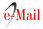Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232On-line version ISSN 2357-3759
Caldasia vol.32 no.1 Bogotá Jan./June 2010
En bosques secos de roble las epífitas vasculares muestran resistencia a la alteración humana, Cordillera Oriental, Colombia
DIEGO HIGUERA
JAN H.D. WOLF
Corporación Sentido Natural, Carrera 70H No. 122 - 98, Apartamento 101, Bogotá, Colombia. higuera@sentidonatural.org
University of Amsterdam, Institute for Biodiversity and Ecosystem Dynamics (IBED), P.O. Box 94248, 1090 GE Amsterdam, The Netherlands. j.h.d.wolf@uva.nl
ABSTRACT
We compared the richness and biomass of vascular epiphytes in six seasonally semi-deciduous oak (Quercus humboldtii) forest fragments of varying structure, using the SVERA protocol. Bromeliads dominated epiphytic vegetation in terms of richness, 10 out of a total of 17 species, and biomass (98%), but overall epiphyte community development was poor in comparison with neotropical wet mountain forests. Epiphyte richness and biomass was similar in all fragments, except one bottom-valley fragment, despite large differences in anthropogenic-induced forest structure. We hypothesize that epiphyte resilience to disturbance in these dry oak forest fragments is due to tolerance of the local epiphyte species to desiccation, overriding micro-climatic differences between forest fragments of different structure.
Key words. Bromeliaceae, deforestation, forest canopy, fragmentation, secondary tropical forest, SVERA, tropical montane forest.
RESUMEN
Comparamos la riqueza y la biomasa de epífitas vasculares en seis fragmentos de bosques estacionales semi-caducifolio de roble (Quercus humboldtii) con diferente estructura, utilizando el protocolo de SVERA. Las bromelias dominaron la vegetación epífita en términos de riqueza, con 10 especies de un total de 17, y con una biomasa del 98%, pero en general el desarrollo de la comunidad de epífitas fue pobre en comparación con bosques húmedos neotropicales. La riqueza de epífitas y la biomasa fue similar en todos los fragmentos, excepto en un fragmento en la parte baja de un valle, a pesar de las grandes diferencias en la estructura de los bosques, inducidas por efectos antropogénicos. Nuestra hipótesis es que la resiliencia de las epífitas locales a los disturbios en estos fragmentos de robledales secos se debe a su tolerancia a la desecación.
Palabras clave. Bromeliaceae, deforestación, dosel, fragmentación, bosques secundarios, bosque andino, SVERA.
Recibido: 14/12/2009
Aceptado: 06/04/2010
INTRODUCTION
For many species of plants and animals, habitat loss and degradation represents the greatest threat to their survival, which is not always recognized because local extinctions may take substantial time to take effect (Kuussaari et al. 2009). Forest degradation typically entails both changes in floristic composition and in structural parameters of the forest such as tree height, tree density and tree basal area, accompanied by alterations of the microclimate (Laurance 2004). In wet tropical forests, epiphytes are particularly vulnerable to forest degradation (Turner et al. 1996). Similar to boreal forests, where epiphytic lichens are used to assess forest ecosystem quality (Liira & Sepp 2009), the epiphyte community in tropical forests may therefore be used to evaluate forest quality, at least in wet areas.
In pristine wet mountain forests, epiphytes are particularly rich in species and abundant (Gentry & Dodson 1987, Benzing 1990), presumably because of high annual rainfall in combination with low seasonality (Kreft et al. 2004). On a regional scale, wet neotropical mountain forests typically contain over 200 and up to 627 (Bussmann 2001) species of epiphytes, reviewed by Wolf and Flamenco-S. (2003). Locally, it is not uncommon to find more than 50 species on just a few host trees, contributing up to more than half of total vascular plant species richness (Kelly et al. 1994)) and green biomass (Hofstede et al. 1993). In these forests, epiphytes play a role in the forest water and nutrient cycles and provide food and habitat for vertebrates, invertebrates and microorganisms alike (Nadkarni and Matelson 1992, Zotz & Andrade 2002). In Colombia, wet mountain oak (Quercus humboldtii) forests are comparably rich in epiphytes. For example, 64 species of epiphytes were found in mixed oak forest in the Cordillera Central (Alzate et al. 2001) and a study on oak forests in the Cordillera Oriental reports 24 orchid and bromeliad species alone (Galeano et al. 2009), suggesting that Q. humboldtii is a suitable host tree species for epiphytes.
In wet forests that are disturbed is some way, however, epiphytes are less prominent. Even though epiphyte response to disturbance has not been extensively studied, ample evidence suggests that the dependent vegetation in wet mountain rain forests is sensitive to habitat changes: isolated remnant trees and secondary forests support significantly less epiphytic abundance, either biomass or number of individuals, and fewer species (Hietz-Seifert et al. 1996, Barthlott et al. 2001, Krömer & Gradstein 2003, Merwin et al. 2003, Werner et al. 2005, Wolf 2005, Köster et al. 2009). Particularly drought sensitive shade epiphytes such as filmy ferns are amongst the first to disappear from the forest upon disturbance, whereas more drought resistant bromeliads may show more resilience (Wolf & Flamenco-S. 2006).
In dry or seasonally dry forests, epiphyte proliferation is much less pronounced compared with wet areas (Gentry & Dodson 1987). Notwithstanding that nearly half of the earth's tropical and subtropical forest is dry forest (Holdridge 1967, Murphy & Lugo 1986), epiphytes in dry forests have been little studied. In Mexico, one study in upland seasonally dry forest reported a mere ten species of epiphytic bromeliads on 63 trees sampled (Reyes-García et al. 2008). The only epiphyte study known to us in dry Andean forests (Ecuador) reported only five bromeliad species and three species of fern (Werner & Gradstein 2009).
With respect to the response of dry-forest epiphytes to anthropogenic disturbance it is still unknown whether dry forest epiphytes are equally susceptible as their wet forest counterparts. Possibly, epiphytes are no good indicators of disturbance in dry forests since it has been suggested that dry forest epiphytes are comparatively disturbance resilient (Werner & Gradstein 2009). To obtain more insight in the behavior of epiphytes in dry forests is important not only because of the large extension of the latter but also because dry forests are especially vulnerable to disturbance (Murphy & Lugo 1995). According to these authors, the development of management strategies for dry forest ecosystems is therefore of the highest priority. If dry forest epiphytes may help to detect disturbance early, appropriate management measures may be taken to avoid further deteriorating of the forest.
In this study, we assess the response of epiphytes to anthropogenic disturbance in a dry semi-deciduous Q. humboldtii forests in Colombia. Q. humboldtii forests are vulnerable to over-exploitation and forest fragmentation because of the considerable economic value of oak trees (Cárdenas-L. & Salinas 2007).
METHODS
Study Area. The study was carried out in the Macanal Reserve (2100 to 2700 m a.s.l.), from hereon called Macanal, approximately 30 km northwest of the capital Bogota (Figure 1).

1 = Plataformas, 2 = Cueva del Oso, 3 = Sendero Alto, 4 = El Encanto, 5 = La Corraleja, 6 = Roble Caído.
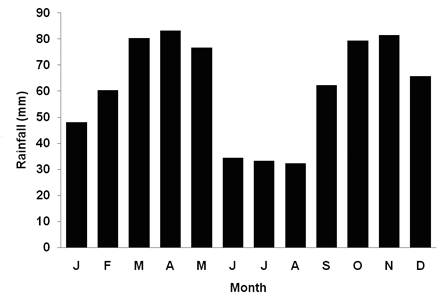
Climate data from Acapulco weather station, situated at less than 500 m from Macanal, shows that average annual temperature is ca. 13 ºC, with little seasonal variation. In contrast, rainfall is bimodal with a first minor dry period from December until March and a second major dry period from June until September when average monthly rainfall is less than 30 mm (Figure 2). Even during wet periods, average monthly rainfall is less than 100 mm; annual precipitation is 738 mm, on average. The corresponding forest type may be classified as montane dry forest, following Holdridge (1967). General water shortage is indicated by the estimated annual potential evapotranspiration ratio, which is above 1.0.
The forest is dominated by oak (Q. humboldtii) trees with an average canopy height of 25 m. In Colombia, oak forests are widely distributed, covering an altitudinal range from 1100 - 3450 m a.s.l. and occurring both in wet and dry areas like Macanal. In wet areas, oak forests are usually mixed with other tree species, e.g. in the genus Weinmannia (Lozano & Torres 1974). In Macanal, oaks form nearly pure stands of forest. Until the 1980's, the logging of oaks and livestock agriculture was intensive at Macanal, resulting in fragments of native oak forest, particularly on the steeper slopes, in various states of degradation, situated in a matrix of pastures, crops and Pinus and Eucalyptus plantations (Figure 1).
Field Sampling. We randomly selected six forest fragments in the Macanal area that varied in forest structure. All selected sites were disturbed by humans to various degrees, evidenced by deviating tree size frequency distributions and canopy height. The Plataformas site was situated in a deep valley whereas Roble Caído bordered a large pasture outside the reserve (Figure 1). Forest structure of each stand was assessed in a 900 m2 plot. All trees were with trunk diameter at breast height (DBH) > 5 cm were identified, counted and their DBH was recorded.
With respect to epiphyte sampling, we implemented the SVERA protocol (Wolf et al. 2009). Epiphyte sampling in SVERA is plotless and tree-based (oaks only). The advantage of SVERA is that epiphyte abundance, in terms of biomass, and species richness may be compared between forests of different structure because differences in the sizes of the sampled trees are controlled for. At each site, ten large trees (DBH > 30 cm) and 25 smaller trees were sampled, equally distributed over five DBH size classes (5-10, 10.1-15, 15.1-20, 20.1-25, 25.1-30 cm). In addition, the height of the tree and the number of forks with a branch diameter > 5 cm was recorded. Larger trees were climbed, using single-rope climbing techniques (Mitchell et al. 2002).
Epiphyte abundance was estimated as dry weight, using an essentially non-destructive sampling method that builds on the previously determined plant size-biomass relationship that was derived from weighing at least ten specimens per size class. For tank bromeliads, size explains biomass very well (Isaza & Betancur 2009). The epiphyte biomass per hectare was calculated from the average tree load per tree trunk diameter class.
Analysis. Differences in epiphyte richness and biomass were compared using ANCOVA, with tree size as the covariate. Tree size was estimated from the tree DBH, height and number of forks (Tree Size = Standardized (Height*DBH) + Standardized (Number of forks). Sites may only be compared in a single ANCOVA if the relationship between Tree Size and richness (or biomass) is the same at all sites, which may be formally tested with an homogeneity assumption test that evaluates the significance of the interaction between sample site and Tree Size (for details, see SVERA, Wolf et al. 2009).
To assess the response of species to differences in forest structure (disturbance), we first reduced the number of forest structure variables using Principal Component Analysis (PCA), thus restraining the number of control variables and avoiding collinearity. A PCA summarizing all forest structure variables yielded three axes that explained 41.8%, 34.6% and 18.7% of total variance, respectively. Next, we entered the three PCA axes in a canonical correspondence analysis (CCA) to evaluate the influence of the forest structure variables on epiphyte species composition. In this analysis, generated ordination axes are constrained to correlate with the entered variables, i.e. the scores on the PCA axes. To test if forest structure had a significant influence on epiphyte species composition, we performed a Monte Carlo significance test of the first axis. Species biomass values were square root transformed.
Finally, we used CCA to test for spatial dependence at the landscape level, following (Borcard et al. 1992). The geographic positions of the sites were used to construct a matrix of spatial variables that are used as explanatory variables in the canonical analysis (Borcard & Legendre 2004). For more details, see SVERA and references therein (Wolf et al. 2009). The analyses were performed using SPSS 15.0.1.1. SpaceMaker2, and CANOCO (ter Braak 1987, 1988).
RESULTS
Forest Structure. Whereas all forest fragments are dominated by oak trees in terms of basal area, the structure of the sampled forests varied much, presumably due to historic differences in the intensity and quality of anthropogenic disturbances (Figure 3, Table 1).
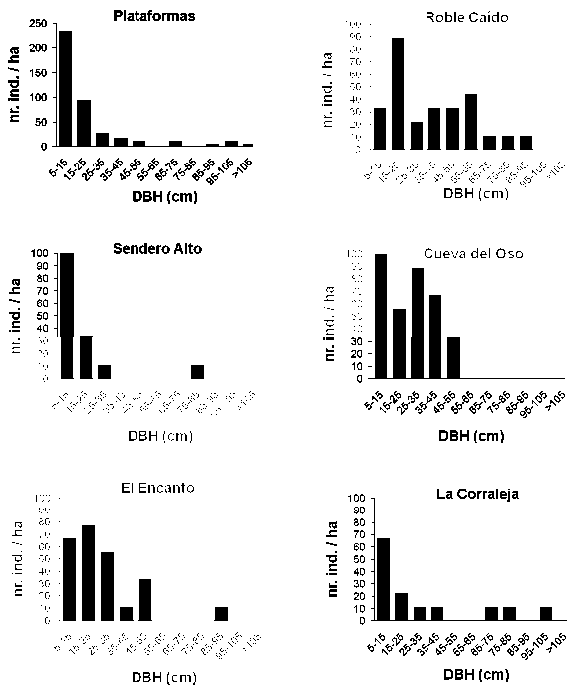
Tree density corresponds to individuals with trunk DBH > 5cm. NP means not present.
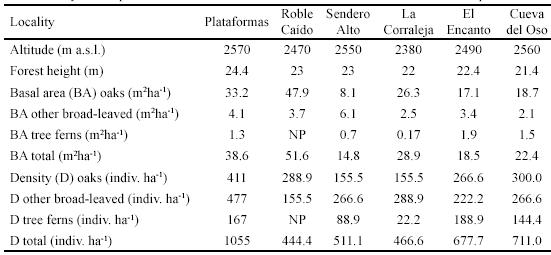
Based on forest height, tree density and basal area, and the trunk size frequency distribution, the Plataformas forest is likely least disturbed. The remaining forests are not easily arranged on a disturbance gradient, even though the absence of large oaks in Sendero Alto suggests heavy selective logging in the past.
Epiphyte richness and biomass. In total, we recorded 17 epiphytes species on the 210 sampled trees (Table 2).
Table 2. Epiphyte species biomass (g dry weight) on 35 oak host trees at the sites.
P: Plataformas, RC: Roble Caído, SA: Sendero Alto, LC: La Corraleja, EE: El Encanto, CO: Cueva del Oso
The epiphyte community was dominated by Bromeliaceae, comprising ten out of 17 species and 98.5% of total biomass. Ferns were represented with 6 species, such as Pleopeltis macrocarpa, a wide-spread ecological generalist that is often found in disturbed forests (Wolf 2005). Of the bromeliads, Tillandsia denudata and T. fendleri were the most common species, contributing > 50% and > 20% to total epiphyte biomass, respectively. Ten species were found in at least 60% of the sites; T. longifolia, T. ovarensis and T. pastensis were exclusive to one site. Epiphyte biomass varied between 22.2 and 547.8 kg per hectare and biomass on the 35 sampled trees between 3.0 and 87.1 kg (Table 3). Highest species richness and biomass was found at Plataformas, the latter due to a dominance of two large clonal tank bromeliads, Tillandsia denudata and T. fendleri.

At all sites, there was a significant (P <0.001) positive linear dependence of epiphyte richness on tree size and also of epiphyte biomass on tree size (Figure 4, Figure 5).
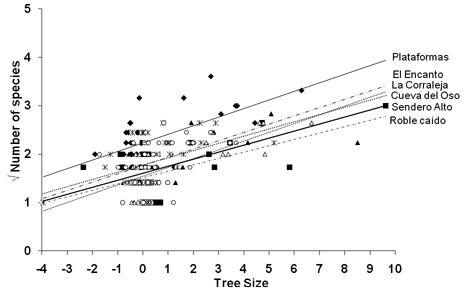
Tree Size = Standardized(Height*DBH) + Standardized(Number of branching points). Plataformas: vrichness =0.18(Tree Size) + 2.23, r2 = 0.36, P < 0.001; El Encanto: vrichness =0.17(Tree Size) + 1.77, r2 = 0.22, P < 0.001; La Corraleja: vrichness = 0.18(Tree Size) + 1.55, r2 = 0.44, P < 0.001; Cueva del Oso: vrichness =0.15(Tree Size) + 1.77, r2 = 0.18, P < 000.1; Sendero Alto: vrichness =0.14(Tree Size) + 1.6, r2 = 0.34, P < 0.001; Roble Caído: vrichness =0.13(Tree Size) + 1.5, r2 = 0.36, P < 0.001.

Tree Size = Standardized(Height*DBH)' + Standardized(Number of branching points). Plataformas: vg biomass =14.8(Tree Size) + 27.7, r2 = 0.56, P < 0.001; El Encanto: vg biomass =5.44(Tree Size) + 16.32, r2 = 0.32, P < 0.001; Cueva del Oso: vg biomass =5.22(Tree Size) + 12.28, r2 = 0.31, P < 0.001; Sendero Alto: vg biomass =4.56(Tree Size) + 9.6, r2 = 0.48, P < 0.001; La Corraleja: vg biomass = 4.31(Tree Size) + 7.06, r2 = 0.58, P < 0.001; Roble Caído: vg biomass =1.82(Tree Size) + 5.5, r2 = 0.42, P < 0.001.
With respect to species richness, all regression lines ran parallel, which was formally tested with a homogeneity assumption test (P=0.91). Therefore it may be concluded that at all sites the same relationship existed between the size of the tree and epiphyte richness. A subsequent ANCOVA with tree size variables as covariates showed that epiphyte richness at Plataformas was significantly higher than at any of the other sites (P<0.001, Table 4). Between all other sites, there were no significant differences in species richness (P>0.01), i.e. the Y-intercept values of the regression lines were the same.
In the upper triangle, epiphyte species richness is compared (columns minus rows) and in the lower triangle epiphyte biomass (rows minus columns). Given are the differences in the adjusted means (i.e. slope elevation). Species richness and biomass (kg dry weight) were square root transformed. Site effect on species richness, F[6,209] = 26,9; P < 0.001, and on epiphyte biomass F[5,174] = 26,3; P < 0.001. Note that for biomass, Plataformas was excluded because the regression coefficient deviated (Figure 5).
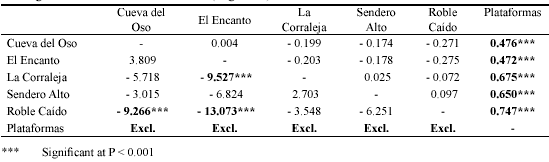
With respect to epiphyte biomass, a similar pattern emerged. Again, values at Plataformas were higher than at any of the other sites (Figure 5). It should be noted, however, that now the slope of the regression line for the Plataformas site was much steeper. The deviant relationship between tree size and biomass at Plataformas was confirmed by the homogeneity test: in the model the interaction between sample site and Tree Size was significant (P<0.001). A comparative analysis of epiphyte biomass between the remaining sites showed that biomass at Roble Caído was always lower than at any of the other sites and significantly so when compared with Cueva del Oso and El Encanto (P<0.001, Table 4).
Species composition. The ordination analysis (CCA) with species data and PCA derived forest structure variables did not generate a first ordination axis that was significantly different from randomly generated axes (Monte Carlo, P=0.14). In other words, we found no evidence that disturbance related to epiphyte species community composition. Similarly, we found no evidence that the position in the landscape influenced species composition (Monte Carlo, P=0.58).
DISCUSSION
Epiphyte species richness and biomass. The epiphyte community at the dry oak forest at Macanal (738 mm/yr) comprised 17 species, which makes it relatively poor in species compared to neotropical wet mountain forests (Wolf & Flamenco-S. 2003). For example, in Colombia, Galeano et al. (2009) report 24 species of epiphytic orchids and bromeliads from Cachalú reserve (3000 mm/yr), growing on 48 oak trees, and 64 species were found on 90 trees in wet mixed oak forests (2000-4000 mm/yr) near Medellin (Alzate et al. 2001). Macanal also contained less species than pine-oak forests in Chiapas, Mexico, (1024 mm/yr) where 74 species were found on 560 trees (Wolf, 2005). The highest number of species, 98 on a single tree and 225 on 6 trees, was recorded in a mountain rain forest of southern Ecuador (Werner et al. 2005). Nevertheless, the Macanal forest is not less rich than Ecuadorian inter-Andean dry forests at Bosque Protector Jerusalén (BPJ, 530 mm/yr) where eight species were found (Werner & Gradstein 2009).
At Macanal, and at BPJ, notably orchids are absent whereas in wet neotropical mountain forests orchids often contribute most to total epiphyte species richness (Gentry & Dodson 1987, Wolf & Flamenco-S. 2003), also in Colombian wet mountain oak forests (Alzate et al. 2001, Galeano et al. 2009). Despite their absence in our inventory, some orchids were observed in the vicinity of Macanal, which raises the question if orchids were gathered from the Macanal forest as ornamental plants. Interviewing local farmers, however, we found no evidence for this activity. Therefore, the absence of orchids in the dry forest at Macanal may be indicative for the susceptibility of the local orchid flora to desiccation.
Bromeliads dominate the epiphyte community at Macanal. Some species, e.g., Tillandsia denudata and T. fendleri, are C3 tank bromeliads that may store water to enhance resilience to desiccation, others are atmospheric CAM species. In terms of species numbers, the epiphyte community is dominated by CAM species. In terms of biomass, however, C3 tank species dominate. Hence, the relative proportion of CAM bromeliads in terms of biomass is not a good predictor of forest dryness.
Overall, epiphyte biomass is relatively low at Macanal, ranging from 22-147 kg/ha (except Plataformas with 548 kg/ha), compared with values reported from wetter (1042 mm/yr) oak forests in Chiapas, Mexico, where most sites (10) supported > 600 kg/ha, and up to 3218 kg/ha, epiphytic biomass (Wolf, 2005). Individual oak trees at Macanal also supported less biomass than oaks in Chiapas, on average 0.68 (SD 0.9) kg and 2.2 (SD 1.7) kg per tree, respectively (35 trees sampled of similar size). Again, low rainfall in Macanal probably accounts for low epiphyte biomass.
Disturbance. Epiphyte response to anthropogenic disturbance could result from a combination of dispersal limitation and changes in the structure of the forest. Logging reduces tree densities and may lead to isolated forest fragments in the landscape. Both type of changes may lead to dispersal limitation in epiphyte populations, albeit at different spatial scales (Wolf 2005, Cascante-Marín et al. 2009), but see Kun et al. (2009). In our study, all sites had a similar number of epiphytes. Thus, at the landscape level we found no evidence for dispersal limitation, despite clear fragmentation of the oak forest (Figure 1). Also, we found no evidence that the distribution of species' abundances in the landscape was spatially dependent (Monte Carlo, P=0.58). Similarly, Werner & Gradstein (2009) found that dispersal was not a key driver of epiphyte diversity in a disturbed dry forest area of northern Ecuador. Possibly, the epiphyte community is less susceptible for dispersal limitation since dominant bromeliads and ferns in this community produce large number of anemochoric propagules. Also, distance between epiphyte communities in the study site fragments was always less than 2 km and negative autocorrelation between epiphyte communities in Mexican oak forests only occurred at larger (>10 km) geographic distances (Wolf 2005).
A change in the structure of the forest may affect its epiphytes in several ways. First, disturbance likely reduces forest complexity and is therefore believed to reduce the number of suitable habitats for epiphytes, at least in wet evergreen closed-canopy forests (Acebey et al. 2003, Benavides et al. 2006, Flores-Palacios & Garcia-Franco 2008). Second, forest disturbance likely brings about microclimatic changes that will result in desiccation stress, similar as found on isolated trees (Hietz-Seifert et al. 1996, Werner et al. 2005).
In view of the above, it is not surprising that in wet forests epiphyte diversity tends to be reduced by anthropogenic disturbance (e.g. Barthlott et al. 2001, Wolf 2005, Flores-Palacios & Garcia-Franco 2008, Köster et al. 2009). However, in dry forests different rules may apply because these forests are less complex to begin with and their species are more tolerant to desiccation.
In our study, there are pronounced differences in forest structural characteristics between the six sites that are likely related to differences in anthropogenic disturbance history (Figure 3, Table 1). The forest at Plataformas was least disturbed, as evidenced by trunk diameter frequency distributions and high tree density, tree basal area and tree height. This forest is situated in a small valley with steep slopes, surrounded by forest, which probably explains reduced logging activity in the past. Even though no microclimatic data are available, field observations confirm that the forest-enclosed valley forest at Plataformas was more humid than any of the other sites.
Low disturbance and high humidity are likely behind the significantly higher epiphyte richness at Plataformas (P<0.001) compared with all the other sites (Figure 4, Table 4 top triangle). This pattern is in agreement with epiphyte distributions amongst disturbed wet forests (Koster et al. 2009). Interestingly, epiphyte richness at all remaining sites was not significantly different (Table 4, top triangle), despite large structural differences (Figure 3, Table 1). Apparently, in this dry oak forest the number of species is not affected by anthropogenic disturbance, similar to observations in dry inter-Andean forests in Ecuador (Werner & Gradstein 2009). Resilience to disturbance is also indicated by the wide distribution of species amongst sites: 11 out of the 14 species in the five sites other than Plataformas were found in at least three sites. In these dry forests, nearly all species are bromeliads that show adaptations to draught such as a tank-morphology, CAM, and dense trichomes. We hypothesize that the dry forest draught-adapted epiphytes are resilient to disturbance because they are tolerant to desiccation stress. Also, it is important to realize that disturbance-induced changes in microclimate in dry forests are relatively minor compared to wet forests since dry forests are more open to begin with, especially during the dry season when trees shed (part of) their leaves.
The forest at Plataformas was not only the most diverse in species, but also contained the most epiphyte biomass (Figure 5, Table 3). Again, we presume that low disturbance and associated high humidity are driving forces. However, it may not be excluded that high biomass is related to the accidental occurrence of the two dominant bromeliad species, Tillandsia denudata and T. fendleri at Plataformas. Individual plants of these clonal large tank-forming species may attain high biomass in comparison to the atmospheric non-tank Tillandsia's that prevail at the other sites. The dominance of these species would also explain why the tree size-epiphyte biomass relationship is different at Plataformas.
Epiphyte biomass at Roble Caído is lower than at any of the other sites. This site borders a large pasture outside the reserve and we attribute low biomass to edge effects, increasing desiccation stress (Lovejoy et al. 1986, Broadbent et al. 2008).
In summary, our study shows that dry forest epiphytes may be more resilient to anthropogenic disturbance than their wet forest counterparts. Hence, dry forest epiphytes are little suitable to assess forest ecosystem quality. However, some caution is in order since we can not exclude the possibility that more vulnerable species have already gone extinct because we have no information about the epiphyte community at Macanal before anthropogenic disturbance. At present, no more logging takes place and it will be interesting to see if epiphyte species richness in the recuperating forest will increase over time.
ACKNOWLEDGMENTS
We thank Corporación Sentido Natural and Macanal Forest Reserve for their support throughout this project. We thank Liliana López and Yolima Pérez for their assistance in data interpretation, Eliana Martínez for her assistance in the laboratory, Nestor García for his help with plant identification and Juliana Rodríguez, Diana Díaz and Camilo Angulo for their help during field work. Funds were provided by Stiching Het Kronendak and Rufford Foundation.
LITERATURE CITED
1. ACEBEY, A., S.R. GRADSTEIN & T. KRÖMER. 2003. Species richness and habitat diversification of bryophytes in submontane rain forest and fallows of Bolivia. Journal of Tropical Ecology 19: 9-18. [ Links ]
2. ALZATE, F., F. CARDONA & R. CALLEJAS. 2001. Diversidad y composición de epífitas vasculares en robledales de Antioquia (Colombia). Actualidades Biológicas 23: 25-31. [ Links ]
3. BARTHLOTT, W., V. SCHMIT-NEUERBURG, J. NIEDER & S. ENGWALD. 2001. Diversity and abundance of vascular epiphytes: a comparison of secondary vegetation and primary montane rain forest in the Venezuelan Andes. Plant Ecology 152: 145-156. [ Links ]
4. BENAVIDES, A.M., J.H.D. WOLF & J.F. DUIVENVOORDEN. 2006. Recovery and succession of epiphytes in upper Amazonian fallows. Journal of Tropical Ecology 22: 705-717. [ Links ]
5. BENZING, D.H. 1990. Vascular Epiphytes. Cambridge University Press. Cambridge. [ Links ]
6. BORCARD, D. & P. LEGENDRE. 2004. SpaceMaker2 - User's guide. Département de sciences biologiques, Université de Montréal. Available from the WWWeb site http://www.fas.umontreal.ca/biol/legendre/. [ Links ]
7. BORCARD, D., P. LEGENDRE & P. DRAPEAU. 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045-1055. [ Links ]
8. BRAAK TER, C.J.F. 1987. The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio 69:69-77. [ Links ]
9. BRAAK TER, C.J.F. 1988. CANOCO - an extension of DECORANA to analyze species-environment relationships. Vegetatio 75: 159-160. [ Links ]
10. BROADBENT, E.N., G.P. ASNER, M. KELLER, D.E. KNAPP & P.J.C.S. OLIVEIRA, JOSE N. 2008. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biological Conservation 141: 1745-1757. [ Links ]
11. BUSSMANN, R.W. 2001. Epiphyte diversity in a tropical Andean forest- Reserva Biológica San Francisco, Zamora-Chinchipe, Ecuador. Ecotropica 7: 43-59. [ Links ]
12. CÁRDENAS-L., D. & N.R. SALINAS. 2007. Libro Rojo de plantas de Colombia. Volumen 4. Especies maderables amenazadas: primera parte. Instituto Amazónico de Investigaciones Científicas SINCHI - Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Bogotá, D.C. [ Links ]
13. CASCANTE-MARÍN, A., N. VON MEIJENFELDT, H. DE LEEUW, J.H.D. WOLF, J.G.B. OOSTERMEIJER & J.C.M. DEN NIJS. 2009. Dispersal limitation in epiphytic bromeliad communities in a Costa Rican fragmented montane landscape. Journal of Tropical Ecology 25: 63-73. [ Links ]
14. FLORES-PALACIOS, A. & J.G. GARCIA-FRANCO. 2008. Habitat isolation changes the beta diversity of the vascular epiphyte community in lower montane forest, Veracruz, Mexico. Biodiversity and Conservation 17: 191-207. [ Links ]
15. GALEANO, C., J.C. LINARES & L.M. CARDENAS. 2009. Algunos aspectos ecologicos de las Bromeliaceae y Orchidaceae epifitas en bosque de Quercus humboldtii, en la Reserva Biologica Cachalú, Santander Colombia. Pp. 10-11 in Resumens II simposio internacional de bosques de roble y ecosistemas asociados. Fundacion Natura Colombia, Universidad Distrital Francisco Jose de Caldas. Bogotá, D.C. [ Links ]
16. GENTRY, A.H. & C.H. DODSON. 1987. Diversity and biogeography of neotropical vascular epiphytes. Annals of the Missouri Botanical Garden 74: 205-233. [ Links ]
17. HIETZ-SEIFERT, U., P. HIETZ & S. GUEVARA. 1996. Epiphyte vegetation and diversity on remnant trees after forest clearance in southern Veracruz, Mexico. Biological Conservation 75: 103-111. [ Links ]
18. HOFSTEDE, R.G.M., J.H.D. WOLF & D.H. BENZING. 1993. Epiphytic mass and nutrient status of an Upper Montane Rain Forest. Selbyana 14: 37-45. [ Links ]
19. HOLDRIDGE, L.F. 1967. Life zone ecology. Tropical Science Center. San José, Costa Rica. [ Links ]
20. ISAZA, C. & J. BETANCUR. 2009. Relationships between biomass and morphological characters of phytotelmata bromeliads in a Colombian upper Andean forest. Caldasia 31: 1-7. [ Links ]
21. KELLY, D.L., E.V.J. TANNER, E.M.N. LUGHADHA & V. KAPOS. 1994. Floristics and biogeography of a rain-forest in the Venezuelan Andes. Journal of Biogeography 21: 421-440. [ Links ]
22. KÖSTER, N., K. FRIEDRICH, J. NIEDER & W. BARTHLOTT. 2009. Conservation of Epiphyte Diversity in an Andean Landscape Transformed by Human Land Use. Conservation Biology 23: 911-919. [ Links ]
23. KREFT, H., N. KOSTER, W. KUPER, J. NIEDER & W. BARTHLOTT. 2004. Diversity and biogeography of vascular epiphytes in Western Amazonia, Yasuni, Ecuador. Journal of Biogeography 31: 1463-1476. [ Links ]
24. KRÖMER, T. & S.R. GRADSTEIN. 2003. Species richness of vascular epiphytes in two primary forests and fallows in the Bolivian Andes. Selbyana 24: 190-195. [ Links ]
25. KUN, A., B. OBORNY & U. DIECKMANN. 2009. Intermediate landscape disturbance maximizes metapopulation density. Landscape Ecology 24: 1341-1350. [ Links ]
26. KUUSSAARI, M., R. BOMMARCO, R.K. HEIKKINEN, A. HELM, J. KRAUSS, R. LINDBORG, E. OCKINGER, M. PARTEL, J. PINO, F. RODA, C. STEFANESCU, T. TEDER, M. ZOBEL & I. STEFFAN-DEWENTER. 2009. Extinction debt: a challenge for biodiversity conservation Trends in Ecology and Evolution 24: 564-571. [ Links ]
27. LAURANCE, W.F. 2004. Forest-climate interactions in fragmented tropical landscapes. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 359: 345-352. [ Links ]
28. LIIRA, J. & T. SEPP. 2009. Indicators of structural and habitat natural quality in boreo-nemoral forests along the management gradient. Annales Botanici Fennici 46: 308-325. [ Links ]
29. LOVEJOY, T.E., R.O. BIERREGAARD, A.B. RYLANDS, J.R. MALCOLM, C.E. QUINTELA, L.H. HARPER, K.S. BROWN, G.V.N. POWELL, H.O. POWELL, R. SCHUBART & M.B. HAYS. 1986. Edge and other effects of isolation on Amazon forest fragments. Pp. 257-325 in M. E. Soule, ed. Conservation biology: the science of scarcity and diversity. Sinauer Associates. Sunderland. [ Links ]
30. LOZANO, G. & J. H. TORRES. 1974. Aspectos generales sobre la distribución, sistemática fitosociológica y clasificación ecológica de los bosques de robles (Quecus) en Colombia. Ecologia Tropical 1: 45-79. [ Links ]
31. MERWIN, M.C., S.A. RENTMEESTER & N.M. NADKARNI. 2003. The influence of host tree species on the distribution of epiphytic bromeliads in experimental monospecific plantations, La Selva, Costa Rica. Biotropica 35: 37-47. [ Links ]
32. MITCHELL, A.W., K. SECOY & T. JACKSON. 2002. The Global Canopy Handbook. Techniques of access and study in the forest roof. Global Canopy Programme. Oxford. [ Links ]
33. MURPHY, P.G. & A.E. LUGO. 1986. Ecology of Tropical Dry Forest. Annual Review of Ecology and Systematics 17: 67-88. [ Links ]
34. MURPHY, P.G. & A.E. LUGO. 1995. Dry forests of Central America and the Caribbean. Pp. 9-34 in S. H. Bullock, H. A. Mooney and E. Medina, ed. Seasonally dry tropical forests. Cambridge University Press. Cambridge. [ Links ]
35. NADKARNI, N.M. & T.J. MATELSON. 1992. Biomass and nutrient dynamics of epiphytic litterfall in a neotropical montane forest, Costa Rica. Biotropica 24(1): 24-30. [ Links ]
36. REYES-GARCÍA, C., H. GRIFFITHS, E. RINCÓN & P. HUANTE. 2008. Niche Differentiation in Tank and Atmospheric Epiphytic Bromeliads of a Seasonally Dry Forest. Biotropica 40: 168-175. [ Links ]
37. TURNER, I.M., K.S. CHUA, J.S.Y. ONG, B.C. SOONG & H.T.W. TAN. 1996. A century of plant species loss from an isolated fragment of lowland tropical rain forest. Conservation Biology 10: 1229-1244. [ Links ]
38. WERNER, F.A. & S.R. GRADSTEIN. 2009. Diversity of dry forest epiphytes along a gradient of human disturbance in the tropical Andes. Journal of Vegetation Science 20: 59-68. [ Links ]
39. WERNER, F.A., J. HOMEIER & S.R. GRADSTEIN. 2005. Diversity of vascular epiphytes on isolated remnant trees in the montane forest belt of southern Ecuador. Ecotropica 11: 21-40. [ Links ]
40. WOLF, J.H.D. 2005. The response of epiphytes to anthropogenic disturbance of pine-oak forests in the highlands of Chiapas, Mexico. Forest Ecology and Management 212: 376-393. [ Links ]
41. WOLF, J.H.D. & A. FLAMENCO-S. 2003. Patterns in species richness and distribution of vascular epiphytes in Chiapas, Mexico. Journal of Biogeography 30: 1689-1707. [ Links ]
42. WOLF, J.H.D. & A. FLAMENCO-S. 2006. Vascular epiphytes and their potential as a conservation tool in pine-oak forests of Chiapas, Mexico. Pp. 375-391 in M. Kappelle, ed. Ecology and conservation of neotropical montane oak forests. Springer-Verlag. Berlin. [ Links ]
43. WOLF, J.H.D., S.R. GRADSTEIN & N.M. NADKARNI. 2009. A protocol for sampling of vascular epiphyte richness and abundance. Journal of Tropical Ecology 25: 107-121. [ Links ]
44. ZOTZ, G. & J.L. ANDRADE. 2002. La ecología y la fisiología de las epífitas y las hemiepífitas. Pp. 271-296 in M. R. Guariguata and G. H. Catan, ed. Ecología y conservación de bosques neotropicales. Libro Universitario Regional del Instituto Tecnológico de Costa. San José, Costa Rica. [ Links ]