Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de la Facultad de Medicina
versión impresa ISSN 0120-0011
rev.fac.med. v.56 n.2 Bogotá abr./jun. 2008
OPINIONES, DEBATES Y CONTROVERSIAS
BIOSYNTHESIS OF MORPHINE:
ITS IMPORTANCE IN PARKINSON´S DISEASE
Biosíntesis de la morfina: su importancia en la enfermedad de Parkinson
Resumen
Se presenta una panorámica tabulada y gráfica de los conocimientos actuales sobre la biosíntesis de la morfina tanto en Papaver somniferum como en los animales. Hacemos un análisis general de dos funciones principales de la morfina en el ser humano y de la importancia de aclarar su biosíntesis para establecer las etapas defectuosas en los enfermos parkinsonianos. Se admite que el daño de las neuronas melánicas de la sustancia negra se produce por neurotoxinas endógenas, metabolitos anormales por cantidad o calidad, resultantes del metabolismo secundario de la dopamina lo cual desencadena la enfermedad de Parkinson idiopática. Deben diseñarse pruebas funcionales que permitan identificar dichos metabolitos en las poblaciones de alto riesgo genético y correlacionarlos con los alelos presentes en ellas. Se concluye que para un diagnóstico preclínico de la enfermedad de Parkinson idiopático es necesario comparar los niveles de morfina proveniente del sistema nervioso central en la sangre de personas normales y en parkinsonianos antes de cualquier tratamiento. Se recomienda un manejo fisiológico y dietético de estas personas (pre-parkinsonianos) antes de la aparición de los signos de la enfermedad.
Palabras clave: enfermedad de Parkinson, diagnóstico precoz, biosíntesis, morfina.
Summary
We sketch present knowledge on the morphine biosynthetic pathway in Papaver somniferum L and in animals. Two main neurophysiological functions of morphine in man are discussed. To explain idiopathic Parkinson´s disease we hypothetically propose the damage of substantia nigra neurons by endogenous neurotoxins resulting from abnormal secondary metabolites of dopamine or similar substances. Metabolic tests should be designed to establish the faulty stages of morphine biosynthesis in parkinsonians. The levels of central nervous system (CNS) produced morphine should be estimated in blood plasma, targeting parkinsonian prone populations (depressed persons), and correlated with abnormal alleles. Early preclinical diagnosed patients should be managed first physiologically and nutritionally.
Key words: Parkinson disease, early diagnosis, biosynthesis, morphine.
Introduction
Early in the XIXth century Sertürner isolated morphine from opium (1), the dried latex of the opium poppy, Papaver somniferum, cultivated for over three thousand years. The only non cultivated plant species reported to synthesize morphine (2) is Papaver setigerum DC, considered in nature as the wild precursor of Papaver somniferum L, but taxono-mically as a subspecies of the latter which has several cultivars1. The cursory proposal, with no supporting experimental data, on the possibility of cultivated lettuce (Lactuca sativa L) being a source of exogenous morphine in milk from women and cows (3) has been disproved (4) without indication by the involved researchers of the horticultural varieties analized, if any in the case of Hazum (3). Lactucarium, the dried latex of wild lettuces, mainly Lactuca virosa L, was used as a mild hypnotic up to the XIXth century, mostly for children. However compositae, as well as gramineal components of hay, also casually supposed to be a source of morphine (3), are botanical families with few alkaloid producing genera.
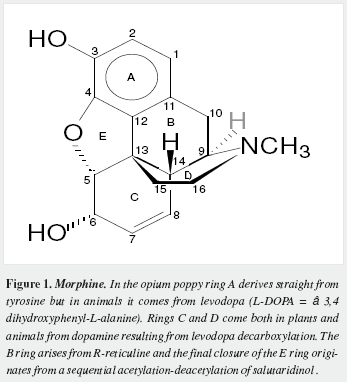
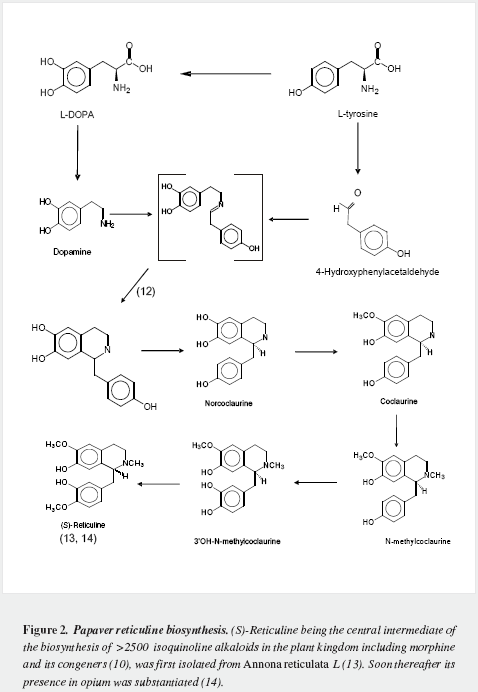
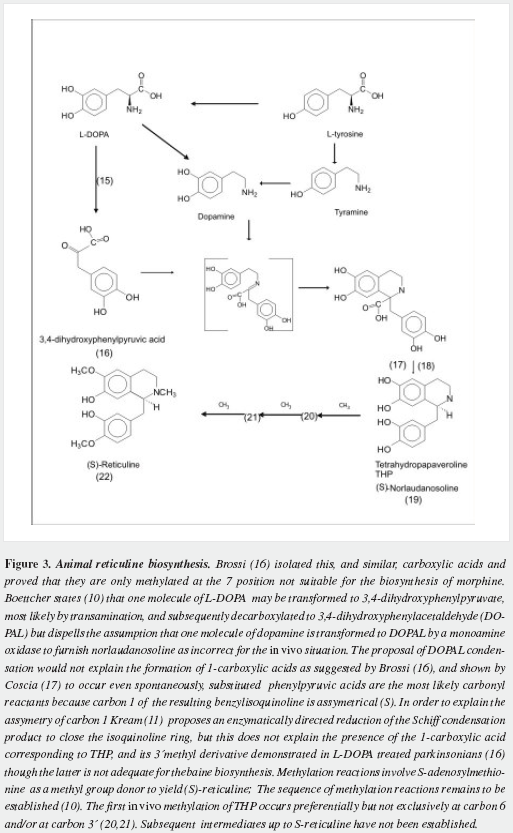
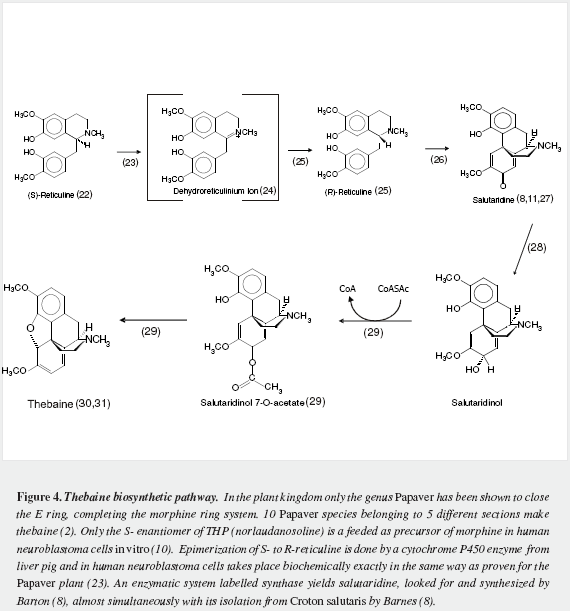
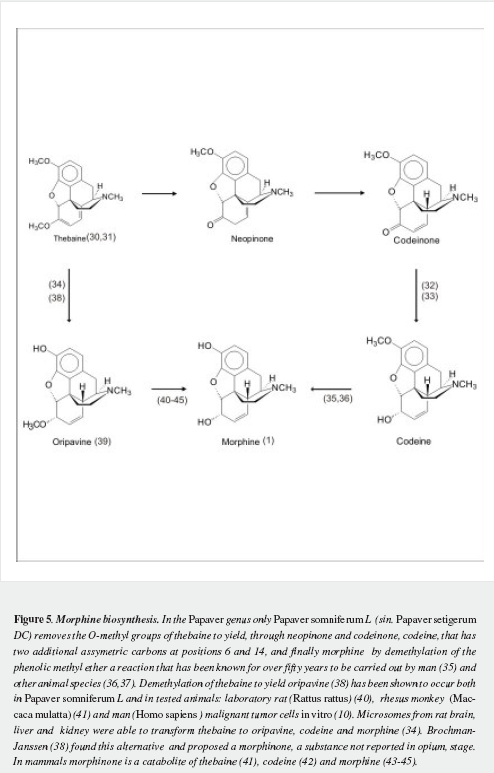
Morphine has a very complex pentacyclic structure with five assymetric carbons (Figure 1), first proposed for its precursor codeine by Guland and Robinson1, and finally proved in the nineteen fifties as summarized by Holmes (5,6). It is the first alkaloid isolated (1) and opened the gate for the discovery of thousands of them in plants in which they are considered a "secondary metabolism". Crucial steps of its biosynthetic pathway were found by Battersby (7) and Barton (8), depicted by Bruneton (9), Poeaknapo (10) and Kream (11) and fully clarified, mainly through the work of Zenk and his research group (12-15) as shown diagrammatically in figures 2-5 (16-45). During the XXth century many efforts were made towards the chemical synthesis of morphine which was achieved by Gates (46), Ginsburg (47) and Beyerman (48) with their coworkers.
Animal morphine endogenous synthesis (AMES)
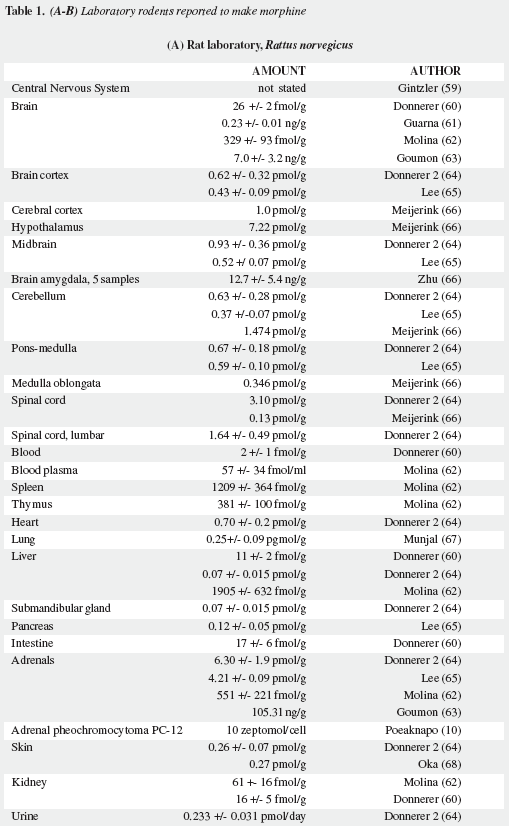
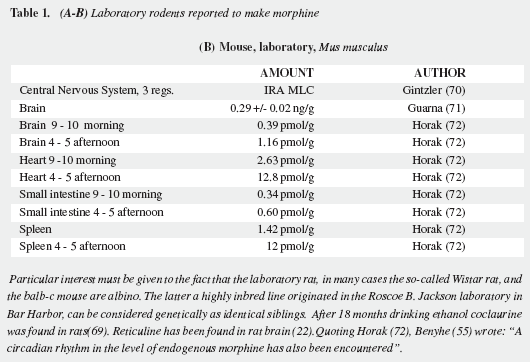
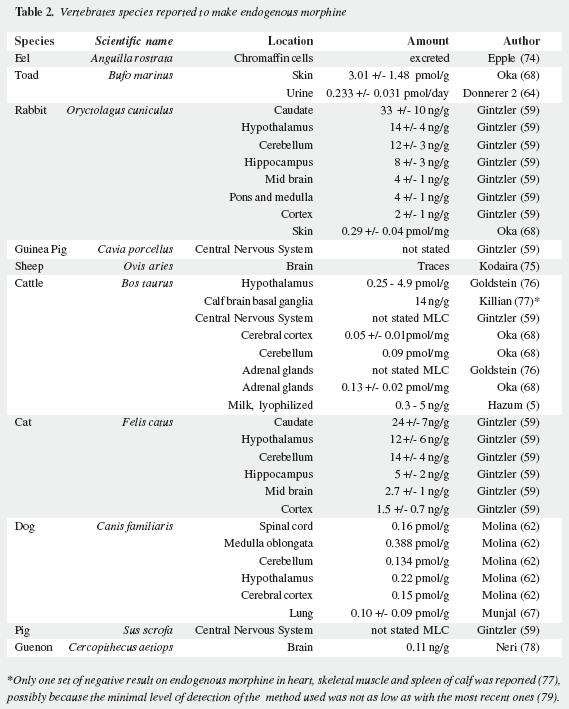
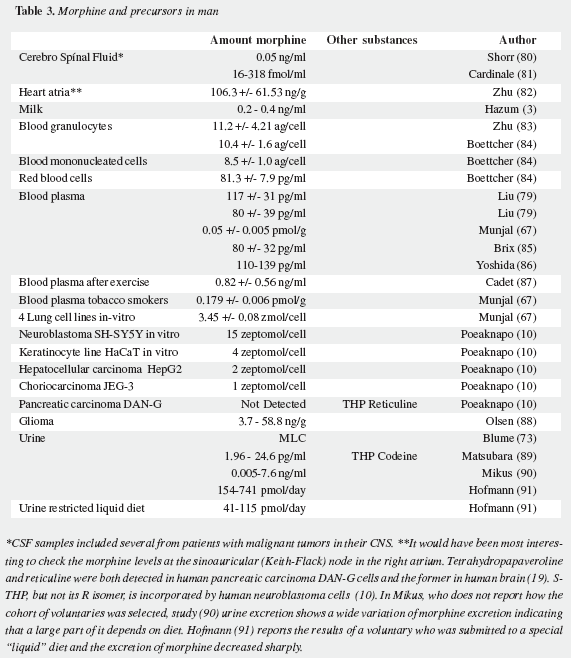
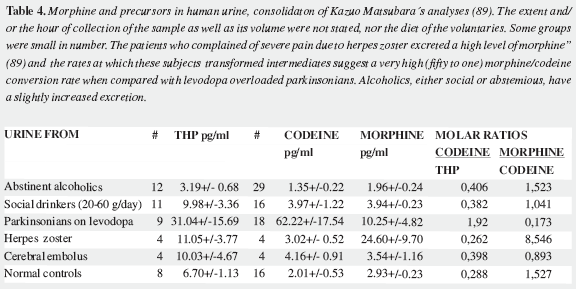
Mavrojanis (49) postulated morphine synthesis by rats, as an explanation of his observations of morphine induced catalepsy. In 1970 Virginia E. Davis speculated on this matter tying the addiction proclivity of alcohol to that of morphine (50-52). Seevers wrongly refuted her on the ground that "unless hypotheses are consonant with known facts they can have no validity" (53) misinterpreting the possible metabolic route. Sourkes (54) insisted in the alternative route leading to noraporphine isomers. Over the last quarter of the XXth century AMES was soundly proved as reviewed by Benyhe (55), Meijerink (56) and Hosztafi (57) and the more recent advances have been summarized by Kream and Stefano (11). Three magnificent reports by Chotima Poeaknapo (4,58), nowadays Böttcher (10), dispelled all possible discussion on the existence of AMES. Morphine was first detected by radioimmunoassays in laboratory rats and mice (Table 1) as well as in human urine (73) and was cautiously labelled by Gintzler "morphine like compound": MLC (59), later characterized as morphine (60). It was also found in a fish, an amphibian, two domestic carnivores, a lagomorph, a third rodent, four artiodactyls (three herbivores and one omnivore), and two primates: a guenon (Table 2) and man (Table 3). In humans morphine has been detected in cells, fluids, tissues, heart atria and tumors. Increased urinary excretion of morphine and two precursors was reported by Kazuo Matsubara (89) in parkinsonian patients treated with levodopa, when compared with normal controls, social and abstemious alcohol drinkers and patients with severe pain due to zona (Table 4); subsequent studies show wide variation in morphine urine excretion in 24 hour samples (90,91) suggesting excretion of both endogenous and exogenous morphine.
Human blood granulocytes make morphine both in vivo and in-vitro (83,84) and it is also present in blood mononuclear and red cells (84) most probably as the result of codeine demethylation (84). SH-SY5Y human neuroblastoma cells provide a model for the complete demonstration of the de novo synthesis of morphine (4) also detected in four normal lung cell (67) and four human cancer lines but not in four human lung cancer cell lines in vitro (67) without addition of precursors, nor in other four human malignant tumors cell lines (4). In human blood plasma significant amounts of
morphine have been found by sensitive (67) and highly sensitive analytical procedures (79), and its presence in cerebrospinal fluid was detected both in normal persons and in severely sick patients ( 80,81).
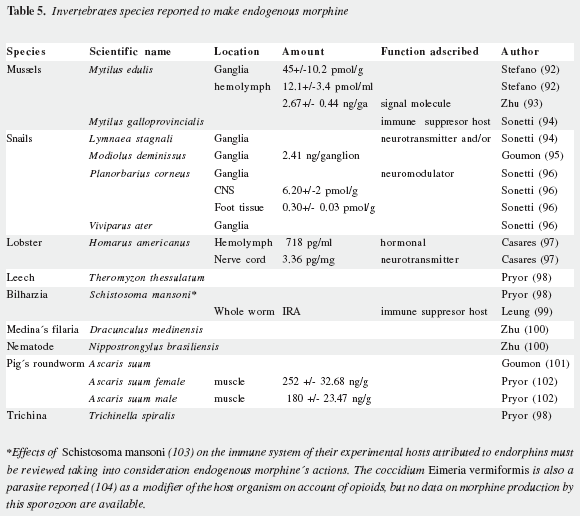
George B. Stefano and his team reported initially the presence of the MLC in invertebrates (92) and they fully characterized it as morphine (93) and over the last decade they have found it in an increasing number of mollusks, crustacea, annelida, nematodes and platyhelminths (Table 5). Numerous assays by this group providing precursors like levodopa and reticuline (106), as well as the increased release by nicotine, cocaine and ethanol (107,108) have been reported to increase morphine production by leukocytes and the edible mussel, Mytilus edulis.
Implications of AMES
During the last 30 years it has been proved beyond any reasonable doubt that animals carry on morphine endogenous synthesis. Kosterlitz (109,110) asked consistently for the physiological relevance of AMES but advanced no suggestions on its role. However, published papers dealing with diverse aspects of endorphins have been extremely numerous while those on AMES were astonishingly scarce and devoted mostly to demonstrate its reality. This situation changed in the last years mostly by the work of Stefano´s group that have incorporated the Morphine Research Society (111). Work over the last fifteen years at the Neuroscience Research Institute of SUNY at Old Westbury has been focused on endogenous morphine action at the molecular and cellular level, while considering the protective action for parasites and infecting agents against their hosts. Several physiological and neuropathological actions have been correlated with AMES in invertebrates (Table 5).
Physiological actions of endogenous morphine
The knowledge accumulated over the last two centuries on the pharmacological effects of morphine (112) indicate two main neurophysiological actions in man, sleep and pain modulation, while its toxicological effects due to abuse clearly show its importance in individual mood and pleasure mechanisms and the withdrawal or "abstinence" syndrome in addicts warns clearly on the frailty of its endogenous synthesis disrupted by massive amounts of exogenous morphine.
SLEEP. Narcosis is one of the main effects of exogenous morphine in humans (112).The relation of endorphins with physiological sleep has been reviewed (113) while no reports on its relation to endogenous morphine could be recovered from the medline data base, where some reports of excretion of exogenous morphine in milk and its action on suckling rats (114) and infants (115) are available. Max (116) speculated on a series of effects of endorphins and casomorphins, active peptides in digested milk: "absorption of opioid peptides generated by peptic digestion of milk may provide a reward to the infant, reinforcing the search for food, increasing mother-infant bonding, and cueing satiation induced sleep", but it is more tempting to suggest that morphine in women´s milk induces both a soothing effect and sleep in their suckling infants without recourse to propose that "perhaps for the infant, periodic exposure to feeding-acquired casomorphins becomes as much a habit as the adult addict´s needle" (116). Moreover the long periods of deep sleep characteristic of infancy (decreasing with age from more than 16 hours a day during the first year to eight hours by puberty) could be determined by high AMES early in life with a decrease with age. In adults both the decrease of tyrosine hydroxylase (TH) activity (117), as well as a hypothetical lowering of AMES would explain the decrease in hours of sleep as well as its lower quality in most ageing persons.
PAIN. Opium and its composite preparations were used since antiquity for pain relief. Its quintessence was sought for that main reason (1) and morphine is, indeed, the most effective, though not devoid of risks, "pain killer". The neurochemical basis for pain have been described (118) and the "activation" of morphine made by leukocytes "may contribute to peripheral pain perception" (119). Guarna (120) has shown the importance of AMES in the modulation of acute thermononiception in mice (121), as well as a neurotransmitter in the central nervous system (CNS) (121). Matsubara demonstrated a high rate of conversion of codeine into morphine in persons with painful herpes zoster (zona) as shown by their rates of excretion (89).
The demonstration of morphine in seven gliomas (88) and in four out of 12 different human malignant tumor lines (10,67) opens a new field of research to look if absence of pain in most human malignant tumors depends on morphine production and monitoring morphine blood levels might be a potential indicator of malignancy. Mammary adenocarcinomas is a particular group to study since mammary glands excrete morphine (5).
Relevance for the understanding of Parkinson´s disease
One hundred and ninety years ago James Parkinson (122) described as suffering from "shaking palsy" six men with a very discapacitating and protracted syndrome characterized by what was known as a triad and Charcot proposed to name it Parkinson´s disease, while discussing both "tremor" and "paralysis" terms (123). The first paragraph of the monography is hereby quoted to back the conclusions of this overview: "The advantages that have been derived from the caution with which hypothetical statements are admitted, are in no instance more obvious than in those sciences which more particularly belong to the healing art. It therefore is necessary, that some conciliatory explanation should be offered for the present publication: in which, it is acknowledged, that mere conjecture takes the place of experiment; and, that analogy is the substitution for anatomical examination, the only sure foundation for pathological knowledge" (122). The clinical course of this insidious, "highly afflictive" (122), condition begins usually with a unilateral tremor of the fingers, the well known movement of "counting coins", which might extend to the arm of the same side and even further to parts of the other limbs. This stage I (124) of Parkinson´s description can last for years. The other components of the motor triad: bradikynesia and rigidity become apparent stepwise to complete the classical case of "idiopathic" Parkinson´s disease (PD). A number of additional malfunctions give an individual pattern according to each patient and each parkinsonian has additional problems in the course of his disease; amongst them the most troubling disorder is a tendency to tumble and fall due to lack of adequate response to obstacles while walking or slipping, a condition aggravated by "a propensity to bend the trunk forwards, and to pass from a walking to a running pace" (122) so that falling can occur even by retropulsion. Poewe (125) distinguishes two main clinical motor groups of parkinsonians: tremor dominant type and rigidity-bradikynetic type, but the presence of a number of additional problems leads him to consider that PD is more than the simplified concept of a motor disorder (126). It is not stated if the less malignant course of the tremulous type before rigidity appears correlates with less severe neuronal lesions and at what specific neural centers.
Etiology
Parkinson (122) deduced from the knowledge at his time that the supposed proximate cause of the disease was located at the upper spinal cord: "By the nature of the symptoms we are taught, that the disease depends on some irregularity in the direction of the nervous influence the injury is rather in the source of this influence"; "this may be the result of injuries of the medulla itself ". Since then facts have accumulated that locate the main disturbance for the motor problems at brain´s basal ganglia, mainly in the pars compacta of the substantia nigra (SN), where the melanized neurons are extremely reduced both in number and in functional activity. Dopamine very low levels are responsible for the most incapacitating signs: rigidity and bradikynesia. Most authors feel that PD cause is "unkown" but recently, however, a large number of facts point to endotoxins and most likely methylated ones as the primary cause of melanized neurons death: 1-benzyltetrahydroisoquinolines have been reported as neurotoxins in laboratory animals (127,128); in humans very interesting reports (129-131) are related to toxins present in leaves of the soursop tree, Annona muricata L, which, used as a tea to sedate and going to sleep, gives an "atypical Parkinson" that reverts on the timely withdrawal of the infusions. Parkinson inducing effects of tetrahydroisoquinolines arising after ethanolic ingestion (132,133) contrast with the lower incidence of PD in alcohol consuming people (134).
Pearce reported aggravation of signs of PD by loading methionine to the diet of parkinsonians on levodopa treatment (135). Several reports on the inducing effect of S-adenosylmethionine in mice (136,137) emphasize the importance of methylation to produce parkinsonism in laboratory animals. Accumulation of methylated derivatives of THP might be noxious to melanized neurons (138,139). Evenmore if methylations are not performed at the correct positions of THP (Figure 3), or are done on analogs with less phenolic groups (formed by reaction of dopamine, tyramine and/or phenylethylamine with deaminated aromatic amino acids (140). Barbeau reported (141) the presence in the urine of 80 percent of his parkinsonians of the "pink spot" that Boulton demostrated to be tyramine (142) and concluded: "the significance of those abnormally large amounts of urinary p-tyramine is not yet understood" and that "many minor spots were observed they were not further investigated". Even methylation at a step unsuitable for building the morphinane ring system, like 1-benzyl,1-carboxytetrahydroisoquinolines (16) at carbon seven, would lead to abnormal metabolic products and it is worth investigating their presence in early parkinsonians by the most adequate methods of analysis, before instituting any pharmacological therapy and/or in the CNS of dead untreated parkinsonians. Methylation has already been postulated as the basis of autointoxication in PD (143). N-methylation has been pointed as a probable altered reaction (144), yet it is not possible at this moment to pinpoint a culprit: we lack knowledge of the exact intermediates involved or of similar condensation products that could damage the melanized neurons. A failure in epimerization from the S to the R form of reticuline must also be considered and ruled out.
A substantial number of reports on the importance of exotoxins in the genesis of Parkinson´s disease have dealt with preneurotoxin MPTP uncovered by Davis (145) and Langston (146) as the agent responsible of an extremely severe form of parkinsonism in young adults. Other chemicals, six hydroxydopamine (147), rotenone (148) and paraquat (149), have shown a similar action. A large number of neurotropic drugs cause iatrogenic "parkinsonisms" mostly "tremulous", and reversible after withdrawing these medications, but 10 percent of akinetic drug induced parkinsonisms are irreversible (150). Toxic parkinsonisms and their experimental models are not identical to PD, yet, by their action on melanized neurons, they provide ways that have been extensively used to look for drugs that would "cure" PD or provide neuroprotection. In a similar attempt in vitro studies mostly with cells are used to study the action of numerous agents in relation to PD (151). Drawing final conclusions from those experiments or from those performed by overloading intermediates of the morphine metabolic system either in vivo and more so in vitro is unsound and evenmore extrapolating them to such a complex organism as the human being. Banati (152) states the need of new methods to study cell biologic processes in real life. Neurochemically PD can be considered far more than "a TH deficiency syndrome of the striatum. TH may be involved in the pathogenesis of PD at several different levels" (117): the resulting extremely low production of dopamine is certainly responsible, but its secondary metabolism has not been considered yet as a most important route in man.
At the cellular level in PD there is an extreme loss of neurons at the pars compacta of the substantia nigra due to death either by apoptosis (153) or other reasons (152). The remaining neurons are less pigmented due to decrease of neuromelanin. Damage of mitochondria has been established (154) that become defective or they lacked genetically the ability to perform certain functions. Oxygen free radicals resulting from "oxidative" deamination have been repeatedly suggested as a factor damaging SN neurons. Arai was unable to detect "dopamine-degrading activity of monoamineoxidase in the neurons of the substantia nigra pars compacta of the rat" (155). In the CNS levodopa amino group is lost by transamination (15) since ammonia, "a deadly neurotoxin" (156), producing oxidative deamination (157,158), is unlikely: nervous tissue does not perform the Krebs-Henseleit urea cycle as done by the liver and other possible reactions are inefficient as clinically proved by the ammonia induced toxicity of valproic acid and hepatic coma.
Genetical bases for PD are widely accepted and this area is receiving close attention. According to Forman (159) genetics should provide "a rational nosology for parkinsonian disorders linked to their molecular and genetic underpinnings". Several abnormal alleles have been found in parkinsonian populations and in "familial parkinsonism" (FP): Abeliovich correlates three mutated genes with mitochondrial pathways and two with intracellular protein inclusions (160). Genome-wide genotyping in PD data suggest to Fung (161) that there is no common genetic variant that exerts a large risk for late-onset Parkinson´s disease in white north americans, but there are five genes in young onset Parkinson. Daniel Kam Yin Chan (162) based on "the association between slow acetylator status and PD" asks for establishing the relevant pathophysiologic mechanisms as well as further exploration on the role of genetic polymorphism in the pathogenesis of PD.
Coffee consumption, alcoholic beverages drinking and tobacco cigarette smoking have been found to correspond with lower incidence of PD (163-165). The possibility of a basic behavior that makes individuals less prone to use this type of stimulation has been postulated (166). The influence of physical exercise correlates also with lower risk (167). Statistically designed studies provide clues to risk factors like midlife adiposity (168) as well as high milk and calcium intake in midlife (169) which may modify PD. It appears that parkinsonians, unaware of their genetical basis, shape their infirmity.
Nosography
Preclinical period: latency
It is generally accepted that before clinical signs appear the damage, as observed by photonic microscopy of the SN neurons, is substantial, estimated at 60-70 percent (170) and that a latency period from the time that neuropathological lesions can be seen to the overt development of signs can last from 3-5 years up to 10-14 years or longer. Abbott (171) quotes the suggestion that excessive daytime sleepiness (EDS) can predate clinical PD and four reports of REM sleep behavior disorder predating an average of 10 to 12 years overt signs. Sleep disturbances are extremely common in parkinsonians and could be abscribed to faulty local morphine synthesis both before and during treatment with levodopa. On account of the slow development of PD, Abe (172) postulates a long-term exposure or accumulation of weak PD-causing substances.
Clinical picture
Parkinson´s original essay (122) describes two of his cases as follows: case I " a gardener, leading a life of remarkable temperance and sobriety was disposed to attribute to his having been engaged for several days on employment of considerable exertion of that limb" and case II "the disease being the consequence of considerable irregularities in his mode of living and particularly of indulgence in spirituous liquors" opening the study of life style in relation to PD. It is admitted that parkinsonians have particular personality traits (173) and very frequently depression (174). Charlton (138) tied this two conditions as follows: "If a common causative mechanism is proposed for PD and depression, a characteristic link between the catecholaminergic neurons that control movements and the neurons that control mood should occur in patients who suffer from PD and depression". Though authors vary in their detailed analysis most accept that depression is a main feature of PD, either preceding it, developíng along its motor malfunctions or the result of treatment. Anxiety as a main feature has been pinpointed by Shiba (175).
Hoehn and Yahr (124) classified the degree of severity of motor problems and numbered after stage I (HY), four additional ones : HY II and III for not too disabled patients and the more severe stages HY IV and V, in the latter the patient being "confined to bed or wheelchair unless aided". In addition several numerical grading systems for PD have been proposed in order to evaluate, either physically, psychologically and/or socially, disease progression and to compare the effect of therapies on its clinical course: UPDRS and its most recent update MDS-UPDRS (176). Depression rating scales have been recently reviewed (177) and the impact on personal activities are presently rated by the SCOPA system (178-180).
Another non motor problem to which much attention has been given recently is sleep. Parkinsonians have many well documented problems both at bedtime and during the day, related or not to medication. Ferreira points to excessive daytime sleepiness (EDS) as well as, "inappropriate, irresistible, unpredictable sudden sleep episodes" (181). EDS prevalence rates in PD vary from one in six up to one in two patients, according to literature quoted by Gjerstad (182) who states: " the disease itself contributes to EDS in patients with PD" but Fabbrini (183) feels that EDS is not present in untreated PD patients.
Prognosis
The last HY stages are hard to sustain both for parkinsonians and for their caregivers. Hely (184) found a mean of 7 years to reach HY stages IV-V. Poewe (185) gives 7.5-9 years to reach stage IV and 10-14 years to stage V. As all human life must come to an end, parkinsonians, after a long protracted course, die mostly by pulmonary infection and/or respiratory terminal failure. Hely (184) found a 38 percent mortality rate during the first 10 years.
Treatment
Pharmacological
Belladona (Atropa belladona L) preparations were used to reduce the tremor, with conflicting results and several anticholinergic agents were tried: biperiden is still in use (186). Degkwitz (187), Birkmayer (188) and Barbeau (189) almost simultaneously found that levodopa releaves dramatically the rigidity and decreases the bradikynesia of parkinsonians, though tremor does not disappear. After a long use of this replacement therapy diminishing response is apparent and the course of the disease continues. Yet the lifespan if not prolonged (184) is more bearable to the treated patients particularly during the initial years of therapy. Levodopa is administered with benserazide or carbidopa, which do not traverse the so-called blood brain barrier (BBB), and act as peripheral inhibitors of dopadecarboxylase. This association lowers systemic undesired effects and permits the use of lower dosages of levodopa. Levodopa is not considered toxic (190) and even ingestion of over 80 grams of levodopa by a 61 years old alcoholic parkinsonian male did not kill the patient but produced a superb mixture of signs and symptoms (191). Setting the moment when treatment with levodopa is imperative as the end-point for selegiline evaluation as protective treatment (192) was disputed (193). Fahn, most wisely, considers levodopa-induced dyskinesias and fluctuations primary reasons for delaying the initiation of levodopa therapy (190) and he does not recommend starting high dosage levodopa at the time of diagnosis. Diskynesias due to levodopa treatment poorly managed have been suppressed by morphine (194,195). Cases of treated parkinsonians falling sleep while driving cars have been reported and some authors believe that all dopamine agonists have sedative effects that lead to hypersomnolence (196).The more serious psychiatric problems in parkinsonians are subsequent to pharmacological treatment (197). Brefel-Courbon and his coworkers have demonstrated an increase of the low pain threshold in PD by levodopa administration to previously untreated parkinsonians (198). Poewe (126) considers that medication does not seem to stop the progression of the disease itself, nor prolong the average rate of life. Additional medicaments have been developed that prolong dopamine action by interfering its catabolism: inhibitors of aminooxidase B, like selegiline, or inhibitors of the catechol ortho-methyl transferase (COMT), like ropirinole and tolcapone. Another approach uses dopaminergic drugs, dopamine agonists, as an alternative or to complement levodopa therapy.
Non pharmacological
Before the use of levodopa supporting measures mainly of nursing care and physical therapy were strongly recommended (199). They are most important in the daily life of patients, particularly in advanced stages of the disease. All facilities should be adapted to the limited motor control of parkinsonians. Diet is a fundamental issue to fill each individual needs and facilitate swallowing, digestion and defecation. Improvement of breathing mechanics is certainly vital.
Prevention
The fact that selegiline prevented the toxic action of MPTP in monkeys (200) led to a cooperative effort to determine if using it as a first medication would delay the need of using levodopa as the most effective, though not devoid of risk, therapy for PD (189). The idea of neuroprotection evolved simultaneously and vitamin E was also tested trying to decrease the action of oxygen free radicals: no final conclusions were reached on account of the basic concept of delaying levodopa treatment (190) but Factor found that vitamin E made less severe the course of the disease in fourteen patients (201). Diets for PD have been centered in lowering protein intake (202) and redistributing its intake (203) since aminoacids compete with levodopa´s absorption. It is clear that preventing the damage to neurones impaired in PD is of the utmost importance. The most recent clinical trial in that sense has been disappointing (204,205) but it must be kept in mind that when tremor appears the neuronal damage at the substantia nigra is high and neuroprotection might have been belated. More recently several authors ask for an early pre-clinical diagnosis, i.e. before classical signs appear, as a mean to help parkinsonians (167,206-209). The correct nutrition for each individual should decrease the severity of the infirmities he is prone to, since "keeping proper diet from early youth can significantly prevent the central neuron damage by different toxic substances" (210) and "eating healthy" (211) is a cornerstone of "wellness medicine" (212) in which AMES fundamental importance must be further and intensively investigated.
Morphine determination
Marsden (213) suggested the existence of: "a long period (perhaps 30 years or more) of nigral degeneration before the appearance of clinical symptoms" and stated: "methods of diagnosing PD at the earliest stage (or even before symptoms are present) should be developed", though "should be started in the drug" is a dubious proposition inasmuch as, according to the presented hypothetical etiology, levodopa is partly metabolized to neurotoxins by parkinsonians. This direction has been pointed to in McNaught´s assertion: "It is therefore necessary to determine the level of isoquinolines derivatives in untreated PD subjects compared with normal individuals to determine the correlation between the levels of isoquinoline derivatives and occurrence of the disease" (214).
No data on morphine levels in blood plasma of untreated, so-called levodopa-naïve, parkinsonians are reported. Yet the indian group at Kerala reports: "morphine was absent in the serum of these (PD) patients" (215), " in serum of patients of PD no morphine was detected" (216); we understand that "absent" and "detected" might mean that the substance looked for could have been present in amounts below the level of detection of their analytical procedures. Pharmacologically untreated parkinsonians is the first group that must be investigated. Subsequently endogenous depressed persons would be another group for study. An extensive review on analytical methods for morphine has been published (217) and can be the basis for selecting the most sensitive, reliable and reproducible way of determining morphine level in blood, taking into account the claim by SUNY Institute for Neuroscience at Old Westbury (85) as a starting point.
Collection of blood samples
Standarization of sample collection is of the utmost importance since definite changes have been shown in human urine excretion depending on diet, alcohol ingestion, and cigarette smoking.
Fasting in rats (65) and in mussels (218) increase their morphine synthesis. Considering the daily variation of morphine levels in diverse mouse organs (72) studies on daily variation of morphine blood levels in humans, keeping in mind the circadian rhythm of each individual (early risers vs late risers) in accordance with the postulated importance of morphine in physiological sleep. Although the reports of Mikus and Hofmann (90,91) do not detail the kind of food their volunteers ingested, the large number of potential animal sources of morphine should exclude their ingestion for a sufficient period of time prior to blood sampling to detect its levels in plasma.
Development of metabolic tests
In addition to nervous tissue morphine is synthesized by many organs of the rat (Table 1) and it is necessary to determine the amount of blood morphine due to CNS production in humans, by loading peripheral decarboxylase inhibitors and checking level of morphine in blood plasma at three moments: before the test, at a correct time after giving them the peripheral dopadecarboxylase inhibitor and after a standardised dose of levodopa.
Sophisticated analytical procedures using labelled precursors should be designed aiming to clarify the methylated metabolites leading from THP to S-reticuline. Thereafter levodopa administration with a decarboxylase inhibitor would permit to study the leaking of those metabolites or the presence of abnormal ones (142). Correlation of the results obtained with those from advanced techniques (219-221) should be carried on.
In addition to the untreated parkinsonians, the above sketched studies should be conducted in selected groups of endogenous depression, which constitutes a most important group of PD patients and of people bearing alleles clearly linked to PD, YOP, FP and similar diseases. If parkinsonians can be diagnosed as such before overt motor signs appear, or whenever the existence of future neurochemical damage seems highly probable, we feel that physical corrective active training should be instituted aiming fundamentally towards an extreme improvement of voluntary control of muscular relaxation, walking dynamics, bilateral movements, strengthening of spinal extensor muscles, etc. The advantage of group management for parkinsonians, before they become a statistical uncertainty, should be wisely practiced in order to fulfill (or even increase) their social needs and promote as much optimism as feasible restoring their "joie de vivre" while feeding them a most attractively presented diet barely covering the basic requirements of essential aromatic aminoacids and methionine, in spite of Bao Ting Zhu´s suggestions (222).
Acknowledgments
The analytical compiler of this hypothetically directed review recognizes with deep, everlasting gratitude, the professional help of his colleagues Armando Carvajal Puyana, Gonzalo Martínez Sanmartín, Francisco Montoya Pardo and Gabriel Osorno Mesa who carried his pre-parkinsonian mother to the old age in which she developed her clinical Parkinson´s disease. As already stated (223) we owe similar feelings to Professor Pablo Lorenzana Pombo for his wise guidance of the levodopa treatment, Professor Hector A. Tejada Hernández (R.I.P.) for his design of physical therapy and Ms. María Teresa Acosta for carrying it on. The author gives a well deserved recognition to his clinical professors (224) who imbued him with a humane attitude towards patients. My recognition is due to the editors of our Revistas Professors Carlos A. Agudelo, Germán Enrique Pérez R. and Edgard Prieto Suárez for their patience and understanding, to Professors Antonio Trías Pujol, Jean Verne, Aaron Bunsen Lerner and Richard Evans Schultes for their lifelong influence, to my classmates Eduardo Arciniegas Álvarez, Maria E. Kane and Hernando Torres Chávez, and particularly to Alejandro Uribe Peralta for reading and commenting the manuscript.
We thank Ingrid Herrera, Yeimi Cárdenas and Lore Ezpeleta Merchán for drawing the Corel figures and Sorne Ezpeleta Merchán for setting the Excel tables and both her and Ms. Ruth Molina Lizcano for the final setting of this paper.
This work could not have been achieved without the open and free access provided by the National Center throng for Biological Information, NLM, NIH, USPHS, in Bethesda (Md), the PubMed database and the most liberal policy for recovery of documents by the Proceedings of the National Academy of Sciences (USA), as well as the Medical Science Monitor and other free access serials. We thank, for their help retrieving articles, the librarians María Elvira Ramírez Castilla and Martha Muñoz, of the Central Library of our University, Luz María Cabarcas of the Universidad Javeriana Library, Imelda Flórez of the Biblioteca Nacional de Salud, María Angeles Langa Langa of the Instituto Cajal, Madrid. Soledad Montero Corredera and Marta Silva Iglesias of the Universidad Complutense Facultad de Farmacia Library and all other persons who contributed to document this review.
José Perea-Sasiaín
(D.M.C) Doctor en Medicina y Cirugía
Universidad Nacional de Colombia
Facultad de Medicina
Correspondencia: josepesa@gmail.com
1. Special thanks to Professor Ryan J. Huxtable for his timely advice on the extent of Guland and Robinson original paper and both to him and botanist José Luis Fernández Alonso for clarifying to me the taxonomical binomial of the opium poppy.
References
1. Huxtable RJ, Schwarz SK. The isolation of morphine-first principles in science and ethics. Mol Interv. 2001;1:189-91.
2. Preininger V. Chemotaxonomy of papaveraceae and fumariaceae. The Alkaloids. 29. Brossi A, ed. Orlando: Academic Press. 1986:1-98.
3. Hazum E, Sabatka JJ, Chang KJ, Findlay JWA, Cuatrecasas P. Morphine in cow and human milk: could dietary morphine constitute a ligand for specific morphine (µ) receptors? Science. 1981;213:1010-2.
4. Poeaknapo C. Mammalian morphine: de novo formation of morphine in human cells. Med Sci Monit. 2005;11:MS6-17.
5. Holmes HL. The morphine alkaloids, I. In The Alkaloids, vol II. Manske RHF, Holmes HL, eds. New York:
Academic Press. 1952: 2-159.
6. Holmes HL, Stork G. The morphine alkaloids, II. In The Alkaloids, vol II. Manske RHF, Holmes HL, eds. New York: Academic Press. 1952: 162-217.
7. Battersby AR, Foulkes DM, Binks R. Alkaloid biosynthesis. Part VIII. Use of optically active precursor for investigations on the biosynthesis of morphine alkaloids. J Chem Soc. 1965:3323-32.
8. Barton DHR, Kirby GW, Steglich W, Thomas GM, Battersby AR, Dobson TA, et al. Investigations on the biosynthesis of morphine alkaloids. J Chem Soc. 1965;2423-38.
9. Bruneton J. Farmacognosia, 2a. Edición. Villar del Fresno A, Carretero Acame E, Rebuelta Lizabe M. trads. Zaragoza: Acribia. 2001:917.
10. Boettcher C, Fellermeier M, Boettcher C, Dräger B, Zenk MH. How human neuroblastoma cells make morphine. Proc Nal Acad Sci USA. 2005;102:8495-500.
11. Kream RM, Stefano GB. De novo biosynthesis of morphine in animal cells-based model. Med Sci Monit. 2006;12:RA207-19.
12. Samanani N, Facchini PJ. Purification and characterization of norcoclaurine synthase.The first committed enzyme in benzylisoquinoline alkaloid biosynthesis in plants. J Biol Chem. 2002;277:33878-83.
13. Gopinath KW, Govindachari TR, Pai BR, Viswanathan N. The structure of reticuline, a new alkaloid from Annona reticulata. Chem Ber. 1959; 92: 776-9.
14. Brochmann-Hanssen E, Furuya T. New opium alkaloid. J Pharm Sci. 1964;53:575.
15. Sandler M, Johnson RD, Ruthven CRJ, Reid JL, Calne DB. Transamination is a major pathway of L-DOPA metabolism following peripheral decarboxylase inhibition. Nature. 1974;247:364-6.
16. Brossi A. Mammalian alkaloids: conversions of tetrahydroisoquinoline-1-carboxylic acids derived from dopamine. Planta Med. 1991;57(Sup 1) S93-100.
17. Coscia CJ, Burke W, Jamroz G, Lasala JM, McFarlane J, Mitchell J, et al. Ocurrence of a new class of tetrahydroisoquinoline alkaloids in L-dopa-treated parkinsonian patients. Nature. 1977;269:617-9.
18. Sandler M, Carter SB, Hunter KR, Stern GM. Tetrahydroisoquinoline alkaloids: in vivo metabolites of L-dopa in man. Nature. 1973;241:439-43.
19. Sango K, Maruyama W, Matsubara K, Dostert P, Minami C, Kawai M, et al. Enantio-selective occurrence of (S)-tetrahydropapaveroline in human brain. Neurosci Lett. 2000;283:224-6.
20. Meyerson LR, Cashaw JL, McMurtrey KD, Davis VE. Stereoselective enzymatic o-methylation of tetrahydropapaveroline and tetrahydroxyberbine alkaloids. Biochem Pharmacol. 1979;28:1745-52.
21. Cashaw JL, Ruchirawat S, Nimit Y, Davis VE. Regioselective o-methylation of tetrahydropapaveroline and tetrahydroberbine in vivo in rat brain. Biochem Pharmacol. 1983;32:3163-9.
22. Zhu W, Ma Y, Cadet P, Yu D, Bilfinger TV, Bianchi E, Stefano GB. Presence of reticuline in rat brain: a pathway for morphine biosynthesis. Brain Res Mol Brain Res. 2003;117:83-90.
23. Hirata K, Poeaknapo C, Schmidt J, Zenk MH. 1,2-dehydroreticuline synthase, the branch point enzyme opening the morphinan biosynthetic pathway. Phytochem. 2004;65:1039-46.
24. Borkowski AR, Horn JS, Rapoport H. Role of 1,2-dehydroreticulium ion in the biosynthetic conversion of reticuline to thebaine. J Am Chem Soc. 1978;100:276-81.
25. De-Eknambul W, Zenk MH. Purification and properties of 1,2-dehydroreticuline reductase from Papaver som-niferum seedlings. Phytochem. 1992;31:813-21.
26. Amann T, Roos PH, Huh H, Zenk MH. Purification and characterization of a cytochrome P 450 enzyme from pig liver, catalyzing the phenol oxidase coupling of R-reticuline to salutaridine, the critical step in morphine biogenesis. Heterocycles. 1995;40:425-40.
27. Weitz CJ, Faull KF, Goldstein A. Synthesis of the skeleton of the morphine molecule by mammalian liver. Nature. 1987;330:674-6.
28. Gerardy R, Zenk MH. Purification and characterization of salutaridine:NADPH 7-oxidoreductase from Papaver somniferum. Phytochem. 1993;34:125-32.
29. Grothe T, Lenz R, Kutchan TM. Molecular characterization of the salutaridinol 7-o-acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum. J Biol Chem. 2001;276:30717-23.
30. Lenz R, Zenk MH. Acetyl coenzyme A:salutaridinol-7-O-acetyltransferase from Papaver somniferum plant cell cultures. The enzyme catalyzing the formation of thebaine in morphine synthesis. J Biol Chem. 1995;270:31091-6.
31. Kodaira H, Lisek CA, Jardine I, Arimura A, Spector S. Identification of the convulsant opiate thebaine in mammalian brain. Proc Natl Acad Sci USA. 1989; 86:716-9.
32. Lenz R, Zenk MH. Purification and properties of codeinone reductase (NADPH) from Papaver somniferum cell cultures and differentiated plants.Eur J Biochem. 1995;233:132-9.
33. Unterlinner B, Lenz R, Kutchan TM. Molecular cloning and functional expression of codeinone reductase: the penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum. Planta J. 1999;18:565-75.
34. Kodaira H, Spector S. Transformation of thebaine to oripavine, codeine and morphine by rat liver, kidney and brain microsomes. Proc Natl Acad Sci USA. 1988;85:1267-71.
35. Mannering GJ, Dixon AC, Baker EM, Asami T. The in vivo liberation of morphine from codeine in man. J Pharmacol Exptl Ther. 1954;111:142-6.
36. Axelrod J. Enzymic conversion of codeine to morphine. J Pharmacol Exptl Ther. 1955;115:259-67.
37. Elison C, Elliott HW. Studies on the enzymatic N- and O-demethylation of narcotic analgesic and evidence for the formation of codeine from morphine in rats and dogs. J Pharmacol Exp Ther. 1964;144:265-75.
38. Nielsen B, Röe J, Brochmann-Hanssen E. Oripavine-a new opium alkaloid. Planta Med. 1983;48:205-6.
39. Brochmann-Hanssen E. A second pathway for the terminal steps in the biosynthesis of morphine. Planta Med. 1984;50:343-5.
40. Mikus G, Somogyi AA, Bochner F, Eichelbaum M. Thebaine O-demethylation to oripavine: genetic differences between two rat strains. Xenobiotica. 1991;21:1501-9.
41. Yamazoe Y, Numata H, Yamagita T. Thebaine metabolites in the urine of rhesus monkeys. Jpn J Pharmacol. 1981;31:433-42.
42. Kumagai Y, Todaka T, Toki S. A new metabolic pathway of morphine: in-vitro and in-vivo formation of morphinone and morphine-glutathione adduct in guinea pig. J Pharmacol Exp Ther. 1990;255:504-10.
43. Ishida T, Yano M, Toki S. In vivo formation of codeinone and morphinone from codeine. Isolation and identification from guinea pig bile. Drug Metabol Dispos. 1991;19:895-9.
44. Toki S, Yamano S. Production of morphinone as a metabolite of morphine and its physiological role. Yakugaku Zasshi. 1999;119:249-67.
45. Todaka T, Ishida T, Kita H, Narimatsu S, Yamano S. Bioactivation of morphine in human liver: isolation and identification of morphinone, a toxic metabolite. Biol Pharm Bull. 2005;28:1275-80.
46. Gates M, Tschudi G. The synthesis of morphine. J Am Chem Soc. 1956;78:1380-93.
47. Elad D, Ginsburg D. Synthesis in the morphine series. Part VI. The synthesis of morphine. J Chem Soc. 1954:3052-6.
48. Beyerman HC, Lie TS, Maat L, Bosman HH, Buurman E, Bijsterveld EJ, et al. A convenient synthesis of codeine and morphine. Rec Trav Chim Pays-Bas. 1976;95:24-5.
49. Mavrojannis M. L´action cataleptique de la morphine chez les rats. Contribution à la théorie toxique de la catalepsie. C R Soc Biol.1903;55:1092-4.
50. Davis VE, Walsh MJ. Alcohol, amines, and alkaloids: a possible biochemical basis for alcohol addiction. Science. 1970;167:1005-7.
51. Davis VE, Walsh MJ. Answer to Seevers letter. Science. 1970;170:1114-5.
52. Davis VE, Walsh MJ, Yamanaka Y. Augmentation of alkaloid formation from dopamine by alcohol and acetaldehyde in vitro. J Pharmacol Exptl Ther. 1970;174:401-412.
53. Seevers MH. Morphine and ethanol physical dependence: a critique of a hypothesis. Science. 1970;170:1113-4.
54. Sourkes TL. Possible new metabolites mediating the actions of L-Dopa. Nature. 1971;229:413-4.
55. Benyhe S. Minireview. Morphine: new aspects in the study of an ancient compound. Life Sci. 1994;55:969-79.
56. Meijerink WJ, Molina PE, Abumrad NN. Mammalian opiate alkaloid synthesis: lessons derived from plant biochemistry. Shock. 1999;12:165-73.
57. Hosztafi S, Fürst Z. Endogenous morphine. Pharmacol Res. 1995;32:15-20.
58. Poeaknapo C, Schmidt J, Brandsch M, Dräger B, Zenk MH. Endogenous formation of morphine in human cells. Proc Natl Acad Sci USA. 2004;101:14091-6.
59. Gintzler AR, Levy A, Spector S. Antibodies as a means of isolating and characterizing biologically active substances: Presence of a non-peptide, morphine-like compound in the central nervous system. Proc Natl Acad Sci USA. 1976;73:2132-6.
60. Donnerer J, Oka K, Brossi A, Rice KC, Spector S. Presence and formation of codeine and morphine in the rat. Proc Natl Acad Sci USA. 1986;83:4566-7.
61. Guarna M, Neri C, Petrioli F, Bianchi E. Potassium-induced release of endogenous morphine from rat brain slices. J Neurochem. 1998;70:147-52.
62. Molina PE, Hashiguchi Y, Meijerinck WJ, Naukam RJ, Boxer R, Abumrad NN. Modulation of endogenous opiate production: effect of fasting. Biochem Biophys Res Commun. 1995;207:312-7.
63. Goumon Y, Bouret S, Casares F, Zhu W, Beauvillain JC, Stefano GB. Lipopolysaccharide increases endogenous morphine levels in rat brain. Neurosci Lett. 2000;293:135-8.
64. Donnerer J, Cardinale G, Coffey J, Lisek CA, Jardine I, Spector S. Chemical characterization and regulation of endogenous morphine and codeine in rat. J Pharmacol Exptl Ther. 1987;242:583-7.
65. Lee CS, Spector S. Changes of endogenous morphine and codeine contents in the fasting rat. J Pharmacol Exp Ther. 1991;257:647-50.
66. Zhu W, Ma Y, Bell A, Esch T, Guarna M, Bilfinger TV, et al. Presence of morphine in rat amygdala: evidence for the mu3 opiate receptor subtype via nitric oxide release in limbic structures. Med Sci Monit. 2004;10:BR433-9.
67. Munjal ID, Minna JD, Manneckjee R, Bieck P, Spector S. Possible role of endogenous morphine and codeine on growth regulation of lung tissue. Life Sci. 1995;57:517-21.
68. Oka K, Kantrowitz JD, Spector S. Isolation of morphine from toad skin. Proc Natl Acad Sci USA. 1985;82:1852-4.
69. Haber H, Roske I, Rottmann M, Georgi M, Melzig MF. Alcohol induces formation of morphine precursors in the striatum of rats. Life Sci. 1997;60:79-89.
70. Gintzler AR, Gershon MD, Spector S. A non-peptide morphine-like compound: immunocytochemical localization in the mouse brain. Science. 1978:199:447-8.
71. Guarna M, Bianchi E, Bartolini A, Ghelardini C, Galeotti N, Bracci L, et al. Endogenous morphine modulates acute thermonociception in mice. J Neurochem. 2002;80:271-7.
72. Horak P, Haberman F, Spector S. Endogenous morphine and codeine in miceeffect of muramyl peptide. Life Sci. 1993;52:PL255-63.
73. Blume AJ, Shorr J, Finberg JPM, Spector S. Binding of the endogenous nonpeptide morphine-like compound to opiate receptors. Proc Natl Acad Sci USA. 1977;74:4927-31.
74. Epple A, Navarro I, Horak P, Spector S. Endogenous morphine and codeine: release by the chromaffin cells of the eel. Life Sci Pharmacol Let. 1993;52:117-21.
75. Kodaira H, Lisek CA, Jardine I, Arimura A, Spector S. Identification of the convulsant opiate thebaine in mammalian brain. Proc Natl Acad Sci USA. 1989;86:716-9.
76. Goldstein A, Barrett RW, James IF, Lowney LI, Weitz CJ, Knipmeyer LL, et al. Morphine and other opiates from beef brain and adrenal. Proc Natl Acad Sci USA. 1985;82:5203-7.
77. Killian AK, Schuster CR, House JT, Sholl S, Connors M, Wainer BH. A non-peptide morphine-like compound from brain. Life Sci. 1981;28:811-7.
78. Neri G, Guarna M, Bianchi E, Sonetti D, Matteucci G, Stefano GB. Endogenous morphine and codeine in the brain of non human primate. Med Sci Monit. 2004;10:MS1-5.
79. Liu Y, Bilfinger TV, Stefano GB. A rapid and sensitive quantitation method of endogenous morphine in human plasma. Life Sci. 1997;60:237-43.
80. Cardinale GJ, Donnerer J, Finck AD, Kantrowitz JD, Oka K, Spector S. Morphine and codeine are endogenous components of human cerebrospinal fluid. Life Sci. 1987;40:301-6.
81. Shorr J, Foley K, Spector S. Presence of a non-peptide morphine-like compound in human cerebrospinal fluid. Life Sci. 1978;23:2057-62.
82. Zhu W, Bilfinger TV, Baggerman G, Goumon Y, Stefano GB. Presence of endogenous morphine and morphine-6-glucuronide in human heart tissue. Int J Mol Med. 2001;7:419-22.
83. Zhu W, Cadet P, Baggerman G, Mantione KJ, Stefano GB. Human white blood cells synthesize morphine: CYP2D6 modulation. J Immunol. 2005;175:7357-62.
84. Boettcher C, Fischer W, Zenk MH. Comment on Human white blood cells synthesize morphine: CYP2D6 modulation. J Immunol. 2006;176:5703-4.
85. Brix-Christensen V, Tonnesen E, Sanchez RG, Bilfinger TV, Stefano GB. Endogenous morphine levels increase following cardiac surgery as part of the antiinflammatory response? Int J Cardiol. 1997;62:191-7.
86. Yoshida S, Ohta J, Yamasaki K, Kamei H, Harada Y, Yahara T, et al. Effect of surgical stress on endogenous morphine and cytokine levels in the plasma after laparoscopoic or open cholecystectomy. Surg Endosc. 2000;14:137-40.
87. Cadet P, Zhu W, Mantione KJ, Rymer M, Dardik I, Reisman S, et al. Cyclic exercise induces anti-inflammatory signal molecule increases in the plasma of Parkinson´s patients. Int J Mol Med. 2003;12:485-92.
88. Olsen P, Rasmussen M, Zhu W, Toennesen E, Stefano GB. Human gliomas contain morphine. Med Sci Monit. 2005;11:MS18-21.
89. Matsubara K, Fukushima S, Akane H, Kobayashi S, Shiono H. Increase urinary morphine, codeine and tetrahydropapaveroline in parkinsonian patient undergoing L-3,4-dihydroxyphenylalanine therapy: a possible biosynthetic pathway of morphine from L-3,4-dihydroxyphenylalanine in humans.J Pharmacol Exp Ther. 1992;260:974-8.
90. Mikus G, Bochner F, Eichelbaum M, Horak P, Somogyi AA, Spector S. Endogenous codeine and morphine in poor and extensive metabolisers of the CYP2D6 (debrisoquine/sparteine) polymorphism. J Pharmacol Exp Ther. 1994;268:546-51.
91. Hofmann U, Seefried S, Schweizer E, Ebner T, Mikus G, Eichelbaum M. Highly sensitive gas chromatographic-tandem mass spectrometric method for the determination of morphine and codeine in serum and urine in the femtomolar range. J Chromatogr B Biomed Sci Appl. 1999;727:81-8.
92. Stefano GB, Digenis A, Spector S, Leung MK, Bilfinger TV, Makman MH, et al. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression.Proc Natl Acad Sci USA. 1993;90:11099-103.
93. Zhu W, Baggerman G, Goumon Y, Casares F, Brownawell B, Stefano GB. Presence of morphine and morphine-6-glucuronide in the marine mollusk Mytilus edulis ganglia determined by GC/MS and Q-TOF-MS. Starvation increases opiate alkaloid levels. Brain Res Mol Brain Res. 2001;88:155-60.
94. Sonetti D, Mola L, Casares F, Bianchi E, Guarna M, Stefano GB. Endogenous morphine levels increase in molluscan neural and immune tissues after physical trauma. Brain Res. 1999;835:137-47.
95. Goumon Y, Casares F, Zhu W, Stefano GB. The presence of morphine in ganglionic tissues of Modiolus deminissus: a highly sensitive method of quantitation for morphine and its derivatives. Brain Res Mol Brain Res. 2001;86:184-8.
96. Sonetti D, Peruzzi E, Stefano GB. Endogenous morphine and ACTH association in neural tissues. Med Sci Monit. 2005;11:MS22-30.
97. Casares FM, McElroy A, Mantione K, Baggermann G, Zhu W, Stefano GB. The american lobster, Homarus americanus, contains morphine that is coupled to nitric oxide release in its nervous and immune tissues: evidence for neurotransmitter and hormonal signaling. Neuro Endocrinol Lett. 2005;26:89-97.
98. Pryor SC, Elizee R. Evidence of opiates and opioid neuropeptides and their immune effects in parasitic invertebrates representing three different phyla: Schistosoma mansoni, Theromyzon tessulatum, Trichinella spiralis. Acta Biol Hung. 2000;51:331-41.
99. Leung MK, Dissous C, Capron A, Woldegaber H, Duvaux-Miret O, Pryor S, et al. Schistosoma mansoni: The presence and potential use of opiate-like substances. Exper Parasitol. 1995;81:208-15.
100. Zhu W, Baggerman G, Secor WE, Casares F, Pryor SC, Fricchione GL, et al. Dracunculus medinensis and Schistosoma mansoni contain opioid alkaloids. Ann Trop Med Parasit. 2002;96:309-16.
101. Goumon Y, Casares F, Pryor S, Ferguson L, Brownawell B, Cadet P, et al. Ascaris suum, an intestinal parasite, produces morphine. J Immunol. 2000;165:339-43.
102. Pryor SC, Henry S, Sarfo J. Endogenous morphine and parasitic helminthes. Med Sci Monit. 2005;11:RA183-9.
103. Kavaliers M, Podesta RB, Hirst M, Young B. Evidence for the activation of the endogenous opiate system in hamsters infected with human blood flukes, Schistosoma mansoni. Life Sci. 1984;35:2365-73.
104. Colwell DD, Kavaliers M. Evidence for involvement of endogenous opioid peptides in altered nociceptive responses of mice infected with Eimeria vermiformis. J Parasitol. 1993;79:751-6.
105. Zhu W, Cadet P, Mantione KJ, Kream RM, Stefano GB. Response to Comment on human white blood cells synthesize morphine: CYP2D6 modulation. J Immunol. 2006;176:5704.
106. Zhu W, Mantione KJ, Shen L, Stefano GB. In vivo and in vitro L-DOPA and reticuline exposure increases ganglionic morphine levels. Med Monit Sci. 2005;11:MS1-5.
107. Zhu W, Mantione KJ, Kream RM, Stefano GB. Alcohol-, nicotine-, and cocaine-evoked release of morphine from human white blood cells: Substances of abuse action converge on endogenous morphine release. Med Sci Monit. 2006;12:BR350-4.
108. Zhu W, Mantione KJ, Casares FM, Cadet P, Kim JW, et al. Alcohol-, nicotine-, and cocaine-evoked release of morphine from invertebrate ganglia: model system for screening drugs of abuse. Med Sci Monit. 2006;12:BR155-61.
109. Kosterlitz HW. Biosynthesis of morphine in the animal kingdom. Nature. 1987;330:606.
110. Kosterlitz HW. Has morphine a physiological function in the animal kingdom? Nature. 1985;317:671-2.
111. Stefano GB. Advances in endogenous morphine. Med Sci Monit. 2005;11:ED1-1.
112. Krueger H, Eddy NB, Sumwalt M. The pharmacology of the opium alkaloids. Publ Health Rep. 1941 Suppl 165: 2 vols. 1-1448, CXL.
113. Kilduff TS, Krilowicz B, Milsom WK, Trachsel L, Wang LC. Sleep and mammalian hibernation: homologous adaptations and homologous processes? Sleep. 1993;16:372-86.
114. Blass EM, Fitzgerald E. Milk-induced analgesia and comforting in 10-day-old rats: opioid mediation. Pharmacol Biochem Behav. 1988;29:9-13.
115. Wittels B, Scott DT, Sinatra RS. Exogenous opioids in human breast milk and acute neonatal neurobehavior: a preliminary study. Anesthesiology. 1990;73:864-9.
116. Max B. This and that: an artefactual alkaloid and its peptide analogs. Trends Pharmacol Sci. 1992;13:341-5.
117. Haavik J, Tosta K. Tyrosine hydroxylase and Parkinson´s disease. Mol Neurobiol. 1998;16:285-309.
118. Kupers R, Kehlet H. Brain imaging of clinical pain states: a critical review and strategies for future studies. Lancet Neurol. 2006;5:1033-44.
119. Salamon E, Esch T, Stefano GB. Pain and relaxation (review). Int J Mol Med. 2006;18:465-70.
120. Guarna M, Bianchi E, Bartolini A, Ghelardini C, Galeotti N, Bracci L, et al. Endogenous morphine modulates acute thermonociception in mice. J Neurochem. 2002;80:271-7.
121. Guarna M, Ghelardini C, Galeotti N, Stefano GB, Bianchi E. Neurotransmitter role of endogenous morphine in CNS. Med Sci Monit. 2005;11:RA190-3.
122. Parkinson J. An essay on the shaking palsy. Sherwood, Neely, Jones. London, 1817. reprinted in J Neuropsychiatry Clin Neurosci. 2002;14:223-36.
123. Goetz CG. Charcot on Parkinson´s disease. Mov Disord. 1986;1:27-32.
124. Hoehn M, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427-42.
125. Poewe W, Gerstenbrand F. Klinische Subtypen der Parkinson-Krankheit. Wien Med Wochenschr. 1986;136:384-7.
126. Poewe W. The need for neuroprotective therapies in Parkinson´s disease: a clinical perspective. Neurology. 2006;66:S2-9.
127. Kotake Y, Tasaki Y, Makino Y, Ohta S, Hirobe M. 1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF. J Neurochem. 1995;65:2633-8.
128. Kotake Y, Yoshida M, Ogawa M, Tasaki Y, Hirobe M, Ohta S. Chronic administration of 1-benzyl-1,2,3,4-tetrahydroisoquinoline, an endogenous amine in the brain, induces parkinsonism in a primate. Neurosci Lett. 1996;217:69-71.
129. Caparros-Lefevbre D, Eibaz A. Caribbean parkinsonism study group. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case control study. Lancet. 1999;354:281-6.
130. Kotake Y, Okuda K, Kamizono M, Matsumoto N, Tanahashi T, Hara H, et al. Detection and determination of
reticuline and N-methylcocularine in the annonaceae family using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:75-8.
131. Lannuzel A, Michel PP, Caparros-Lefebvre D, Abaul J, Hocquemiller R, Ruberg M. Toxicity of Annonaceae for dopaminergic neurons: potential role in atypical parkinsonism in Guadeloupe. Mov Disord. 2002;17:84-90.
132. Antkiewicz-Michaluk L, Krygowska-Wajs A, Szczudlik A, Romañska I, Vetulani J. Increase in salsolinol level in the cerebrospinal fluid of parkinsonian patients is related to dementia: advantage of a new high-performance liquid chromatography method. Biol Psychiatry. 1997;42:514-8.
133. Collins MA. Alkaloids, alcohol and Parkinson's disease. Parkinsonism Relat Disord. 2002;8:417-22.
134. Byles J, Young A, Furuya H, Parkinson L. A drink to healthy aging: The association between older women's use of alcohol and their health-related quality of life. J Am Geriatr Soc. 2006;54:1341-7.
135. Pearce LA, Waterbury LD. L-methionine: a possible levodopa antagonist. Neurology.1974;24:840-1.
136. Charlton CG, Crowell B Jr. Parkinson´s disease-like effects of S-adenosyl-L-methionine: effects of L-DOPA. Pharmacol Biochem Behav. 1992;43:423-31.
137. Charlton CG, Mack J. Substantia nigra degeneration and tyrosine hydroxylase depletion caused by excess S-adenosylmethionine in the rat brain. Support for an excess methylation hypothesis for parkinsonism. Mol Neurobiol. 1994;9:149-61.
138. Charlton CG, Crowell B Jr. Effects of dopamine metabolites on locomotor activity and on the binding of dopamine: relevance to the side efects of L-dopa. Life Sci. 2000,66:2159-71.
139. Gluck MR, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson's disease and catecholamine-associated diseases. J Neurochem. 2004;91:788-95.
140. Lasala JM, Coscia CJ. Accumulation of a tetrahydroisoquinoline in phenylketonuria. Science. 1979;203:283-4.
141. Barbeau A. The «pink spot», 3,4-dimethoxyphenylethylamine and dopamine. Relationship to Parkinson's disease and to schizophrenia. Rev Can Biol. 1967;26:55-79.
142. Boulton AA, Pollitt RJ, Majer JR. Identity of a urinary «pink spot» in schizophrenia and Parkinson's disease. Nature.1967;215:132-4.
143. Williams AC, Ramsden DB. Autotoxicity, methylation and a road to the prevention of Parkinson´s disease. J Clin Neurosci. 2005;12:6-11.
144. Matsubara K, Aoyama K, Suno W, Awaya T. N-methylation underlying Parkinson´s disease. Neurotoxicol Teratol. 2002;24:593-8.
145. Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM et al. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1:249-54.
146. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonisms in humans due to a product of meperidine-analog synthesis. Science. 1983;239:979-80.
147. Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson´s disease. Neurotox Res. 2007;11:151-67.
148. Hoglinger GU, Oertel WH, Hirsch EC. The rotenone model of parkinsonism-the five years inspection. J Neural Transm Suppl. 2006;(70):269-72.
149. Dinnis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Sánchez Navarro A, Bastos ML, et al. Paraquat exposure as an etiological factor of Parkinson´s disease. Neurotoxicol. 2006;27:1110-22.
150. Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf. 2006;5:759-71.
151. Lee JJ, Kim YM, Park HD, Kang MH, Hong JT, Lee MK. Aggravation of L-DOPA-induced neurotoxicity by tetrahydropapaveroline in PC12 cells. Biochem Pharmacol. 2003;66:1787-95.
152. Banati RB, Blunt S, Graeber MB. What does apoptosis have to do with Parkinson´s disease. Mov Disord. 1999;14:384-5.
153. Hirsch EC, Hunot S, Faucheux B, Agid Y, Mizuno Y, Mochizuki H et al. Dopaminergic neurons degenerate by apoptosis in Parkinson´s Disease. Mov Disord. 1999;14:383-4.
154. BenderA, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, et al. High level of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515-7.
155. Arai R, Horiike K, Hasegawa Y. Dopamine-degrading activity of monoamino oxidase is not detected in the
neurons of the substantia nigra pars compacta of the rat. Brain Res. 1998;812:275-82.
156. Cohn RM, Roth KS. Hyperammonemia, bane of the brain. Clin Pediatr. 2004;43:893-9.
157. Cohen G. The pathobiology of Parkinson´s disease: biochemical aspects of dopamine neuron senescence. J Neural Transm Suppl. 1983;(19):89-103.
158. Riederer P, Konradi C, Hebenstreit Youdim MBH. Neurochemical perspectives to the function of monoamine oxidase. Acta Neurol Scand. 1989;126:41-5.
159. Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson´s disease: looking for the way out of a quackmire. Neuron. 2005;47:479-82.
160. Abeliovich A, Beal MF. Parkinson genes: culprits and clues. J Neurochem. 2006;99:1062-72.
161. Fung HC, Scholz S, Matarin M, Simón-Sánchez J, Hernández D, Britton A, et al. Genome-wide genotyping in Parkinson´s disease and neurologically normal controls; first stage analysis and public release of data. Lancet Neurology. 2006;5:911-6.
162. Chan DK, Lam MK, Wong R, Hung WT, Wilcken DE. Strong association between N-acetyltransferase 2 genotype and PD in Hong Kong Chinese. Neurology. 2003;60:1002-5.
163. Rocca WA, Maraganore DM, Benedetti MD. Smoking, alcohol, and coffee consumption preceding Parkinson's disease. Neurology. 2001;56:984-5.
164. Deleu D. Smoking, alcohol, and coffee consumption preceding Parkinson's disease. Neurology. 2001;56:984.
165. Hernan MA, Chen H, Schwarzschild MA, Ascherio A. Alcohol consumption and the incidence of Parkinson's disease. Ann Neurol. 2003;54:170-5.
166. Benedetti MD, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. 2000;55:1350-8.
167. Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and risk of Parkinson's disease: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77:1318-22.
168. Abbott RD, Ross GW, White LR, Nelson JS, Masaki KH, Tanner CM, et al. Midlife adiposity and the future risk of Parkinson's disease. Neurology.. 2002;59:1051-7.
169. Park M, Ross GW, Petrovitch H, White LR, Masaki KH, Nelson JS et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology. 2005;64:1047-51.
170. Becker G, Muller A, Braune S, Buttner T, Benecke R, Greulich W, et al. Early diagnosis of Parkinson´s disease. J Neurol. 2002;249 Suppl3:III/40-8.
171. Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442-6.
172. Abe K, Saitoh T, Horiguchi Y, Utsunomiya I, Taguchi K. Synthesis and neurotoxicity of tetrahydroisoquinoline derivatives for studying Parkinsons´s disease. Biol Pharm Bull. 2005;28:1355-62.
173. Todes CJ, Lees AJ. The pre-morbid personality of patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1985;48:97-100.
174. Mayeux R, Stern Y, Williams JB, Cote L, Frantz A, Dyrenfurth I. Clinical and biochemical features of depression in Parkinson's disease. Am J Psychiatry. 1986;143:756-9.
175. Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov Disord. 2000;15:669-77.
176. Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41-7.
177. Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord. 2007;22:1077-92.
178. Marinus J, Viser M, Martínez-Martín P, van Hilten JJ, Stiggelbout A. A short psychosocial questionnaire for patients with Parkinson´s disease: the SCOPA-PS. J Clin Epidemiol. 2003;56:61-7.
179. Marinus J, Visser M, Stiggelbout AM, Rabey JM, Martinez-Martin P, Bonuccelli U, et al. A short scale for the assessment of motor impairments and disabilities in Parkinson's disease: the SPES/SCOPA. J Neurol Neurosurg Psychiatry. 2004;75:388-95.
180. Martinez-Martin P, Cubo-Delgado E, Aguilar-Barbera M, Bergareche A, Escalante S, Rojo A, et al. Estudio piloto sobre una medida específica para los trastornos del sueño de la enfermedad de Parkinson SCOPA-sueño. Rev Neurol. 2006;43:577-83
181. Ferreira JJ, Desboeuf K, Galitzky M, Thalamas C, Brefel-Courbon C, Fabre N, et al. Sleep disruption, daytime somnolence and sleep attacks' in Parkinson's disease: a clinical survey in PD patients and age-matched healthy volunteers. Eur J Neurol. 2006;13:209-14.
182. Gjerstad MD, Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology. 2006;67:853-8.
183. Fabbrini G, Barbanti P, Aurilia C, Vanacore N, Pauletti C, Meco G. Excessive daytime sleepiness in de novo and treated Parkinson´s disease. Mov Disord. 2002;17:1026-30.
184. Hely MA, Morris JG, Traficante R. O´Sullivan DJ, Williamson PM. The Sydney Multicenter Study of Parkinson's disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry. 1999;67:300-7.
185. Poewe W. The need for neuroprotective therapies in Parkinson´s disease: a clinical perspective. Neurology 2006;66:S2-9.
186. Brocks DR. Anticholinergic drugs used in Parkinson's disease: An overlooked class of drugs from a pharmacokinetic perspective. J Pharm Pharm Sci. 1999;2:39-46.
187. Degkwitz R, Frowein R, Kulenkampff C, Mohs U. On the effects of L-dopa in the man and their modification by reserpine, chlorpromazine, iproniazide and vitamin B6. Klin Wochenschr. 1960;38:120-3.
188. Birkmayer W, Hornykiewicz O. Der L-3,4-dioxyphenylalanin (DOPA)-Effect bei der Parkinson-Akinese. Wien Klin Wochenschr. 1961;73:787-8.
189. Barbeau A. L-Dopa therapy in Parkinson´s disease. A critical review of nine years experience. Can Med Assoc J. 1969;101:791.80.
190. Fahn S, and the Parkinson Study Group. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252 Suppl 4:IV37-IV42.
191. Hoehn MM, Rutledge CO. Acute overdose with levodopa. Neurology. 1975;25:792-4.
192. Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson´s disease. New Engl J Med. 1989;321:1364-71.
193. Landau WM. Clinical neuromythology IX. Pyramid sale in the bucket shop: DATATOP bottoms out. Neurology. 1990;40:1337-9.
194. Berg D, Becker G, Reiners K. Reduction of diskynesia and induction of akynesia induced by morphine in two parkinsonian patients with severe sciatica. J Neural Transm. 1999;106:725-8.
195. Berg D, Becker G, Naumann M, Reiners K. Morphine in tardive and idiopathic dystonia (short communication). J Neural Transm. 2001;108:1035-41.
196. Ferreira JJ, Galitzky M, Montastruc JL, Rascol O. Sleep attacks and Parkinson´s disease treatment. Lancet. 2000;355:1333-4.
197. Lauterbach EC. The neuropsychiatry of Parkinson's disease and related disorders. Psychiatr Clin North Am. 2004;27:801-25.
198. Brefel-Courbon C, Payoux P, Thalamas C, Ory F, Quelven I, Chollet F, et al. Effect of levodopa on pain threshold in Parkinson's disease: a clinical and positron emission tomography study. Mov Disord. 2005;20:1557-63.
199. Denny-Brown D. Paralysis Agitans. In Cecil Textbook of Medicine. Philadelphia: Saunders; 1964. p.1605-8.
200. Cohen G, Pasik P, Cohen B, Leist A, Mytilineou C, Yahr MD. Pargyline and deprenyl prevent the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in monkeys. Eur J Pharmacol. 1984;106:209-10.
201. Factor SA, Sánchez-Ramos JR, Weiner WJ. Vitamin E therapy in Parkinson´s disease. Adv Neurol. 1990;53:457-60.
202. Riley D, Lang AE. Practical application of a low-protein diet for Parkinson´s disease. Neurology. 1988;38:1026-31.
203. Pincus JH, Barry KM. Protein redistribution diet restores motor function in patients with dopa-resistant "off" periods. Neurology. 1988;38:481-3.
204. Lang AE. Neuroprotection in Parkinson´s disease: and now for something completely different? Lancet Neurology. 2006;5:990-1.
205. Olanow CW, Schapira AHV, LeWitt PA, Kieburtz K, Sauer D, Olivieri G, et al. TCH346 as a neuroprotective
drug in Parkinson´s disease: a double blind, randomised, controlled trial. Lancet Neurol. 2006;5:1013-20.
206. Waldmeier P, Bozyczko-Coyne D, Williams M, Vaught JL. Recent clinical failures in Parkinson´s disease with apoptosis inhibitors underline the need for a paradigm shift in drug discovery for neurodegenerative diseases. Biochem Pharmacol. 2006;72:1197-206.
207. DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830-4.
208. Berendse HW, Ponsen MM. Detection of preclinical Parkinson´s disease along the olfactory tract. J Neural Transm Suppl. 2006;(70):321-5.
209. Siderowf A, Stern MB. Preclinical diagnosis of Parkinson´s disease: are we there yet? Curr Neurol Neurosci Rep. 2006;6:295-301.
210. Antkiewicz-Michaluk L. Endogenous risk factors in Parkinson´s disease: dopamine and tetrahydroisoquinolines. Pol J Pharmacol. 2002;54:567-72.
211. Esch T. Kim JW, Stefano GB. Neurobiological implications of eating healthy. Neuro Endocrinol Lett. 2006;27:21-33.
212. Stefano GB. Endogenous morphine: a role in wellness medicine. Med Sci Monit. 2004;10:ED5.
213. Marsden CD. Parkinson´s disease. Lancet. 1990;335:948-52.
214. McNaught KS, Carrupt PA, Altomare C, Cellamare S, Carotti A, Testa B, et al. Isoquinoline derivatives as endogenous neurotoxins in the aetiology of Parkinson´s disease. Biochem Pharmacol. 1998;56:921-33.
215. Kurup RK, Kurup PA. Hypothalamic digoxin-mediated model for Parkinson's disease. Int J Neurosci. 2003;113:515-36.
216. Arun P, Ravikumar A, Leelamma S, Kurup PA. Endogenous alkaloids in the brain of rats loaded with tyrosine/ tryptophan & in the serum of patients of neurodegenerative & psychiatric disorders. Ind J Med Res. 1998;107:231-8.
217. Bosch ME, Sánchez AR, Rojas FS, Ojeda CB. Morphine and its metabolites: analytical methodologies for its determination. J Pharm Biomed Anal. 2007;43:799-815.
218. Zhu W, Baggerman G, Goumon Y, Casares F, Brownawell B, Stefano GB. Presence of morphine and morphine-6-glucuronide in the marine mollusk Mytilus edulis ganglia determined by GC/MS and Q-TOF-MS. Starvation increases opiate alkaloid levels. Brain Res Mol Brain Res. 2001;88:155-60.
219. Filippi L, Manni C, Pierantozzi M, Brusa L, Danieli R, Stanzione P, et al. 123I-FP-CIT in progressive supranuclear palsy and in Parkinson's disease: a SPECT semiquantitative study. Nucl Med Commun. 2006;27:381-6.
220. Marshall VL, Patterson J, Hadley DM, Grosset KA, Grosset DG. Successful antiparkinsonian medication withdrawal in patients with Parkinsonism and normal FP-CIT SPECT. Mov Disord. 2006;21:2247-50.
221. Matsui H, Nishinaka K, Oda M, Hara M, Komatsu K, Kubori T, et al. Excessive daytime sleepiness in Parkinson disease: a SPECT study. Sleep. 2006;29:917-20.
222. Zhu BT. CNS dopamine oxidation and catechol-O-methyltransferase: importance in the etiology, pharmacotherapy, and dietary prevention of Parkinson´s disease. Int J Mol Med. 2004;13:343-53.
223. Perea-Sasiaín J. Reflexiones sobre la biosíntesis de la morfina y el Parkinson idiopático. Rev Salud Pública. 2007;9:308-14.
224. Gómez González J. Bodas de oro profesionales (1955-2005) Promoción 1955. Facultad de Medicina, Universidad Nacional de Colombia. Medellín: Universidad Cooperativa de Colombia; 2005.